Solution Brief
Features
Business Challenges
During the product research and development lifecycle, a large variety of documents that describe the product design and definition, analysis plans, results, and safety and summary reports are developed and submitted to regulatory health agencies. Today, the information created, shared, reviewed, and approved is captured in unstructured documents developed throughout the product research and development phases, which can span ten or more years. Many of these documents are made up of reusable subcomponents that are generated by experts, scientists, and systems, increasing both the complexity, volume, and consistency of information. The ability to trace the information through the entire product development cycle is also difficult.
To ensure quality, consistency, and improved efficiencies, structuring the information within documents into meaningful components and reusing information within and across documents with full traceability is essential. Structuring the information for reuse and traceability also aids in making key decisions during the authoring, review, and approval phases. Structuring content also promotes easier to track component level lifecycles and publishing of appropriate content components into required formats for downstream processes, systems, or applications.
The InteliNotion Solution
InteliNotion delivers a patented, revolutionary Structured Content Authoring, Component Content Management and Content Governance Cloud native SaaS solution built on the latest Web technologies that provides a set of unique business capabilities, introducing a sophisticated yet user friendly approach to structured content authoring and component content management.
Overall, structured content authoring and dossier (collection of related documents for a business purpose) management have fundamentally the same issues: content planning, component level lifecycles, reuse, ease of use, and traceability. The InteliNotion platform capabilities address the key pain points in the authoring, review, and approval processes with a user-friendly and friction free solution approach.
Key pain points described by many of our clients include:
- Manual copy/paste of content within documents
- Difficult and time-consuming process to find correct content for reuse while authoring.
- Manual reuse of content using copy/paste across documents
- Inability to track what content was used in what document and/or in what dossier or submission.
- Inability to determine impact of change on up or downstream documents or dossiers.
- Manual and intensive downstream document generation and reporting
- Cumbersome user tagging leads to low data quality.
- Business processes do not quite fitting the tool or system, often resulting in many workarounds.
The InteliNotion platform supports configurable information models, reuse policies, component level lifecycle management, business rules, and templates for various business information and functional needs.
The following diagram illustrates the capabilities of the overall solution.
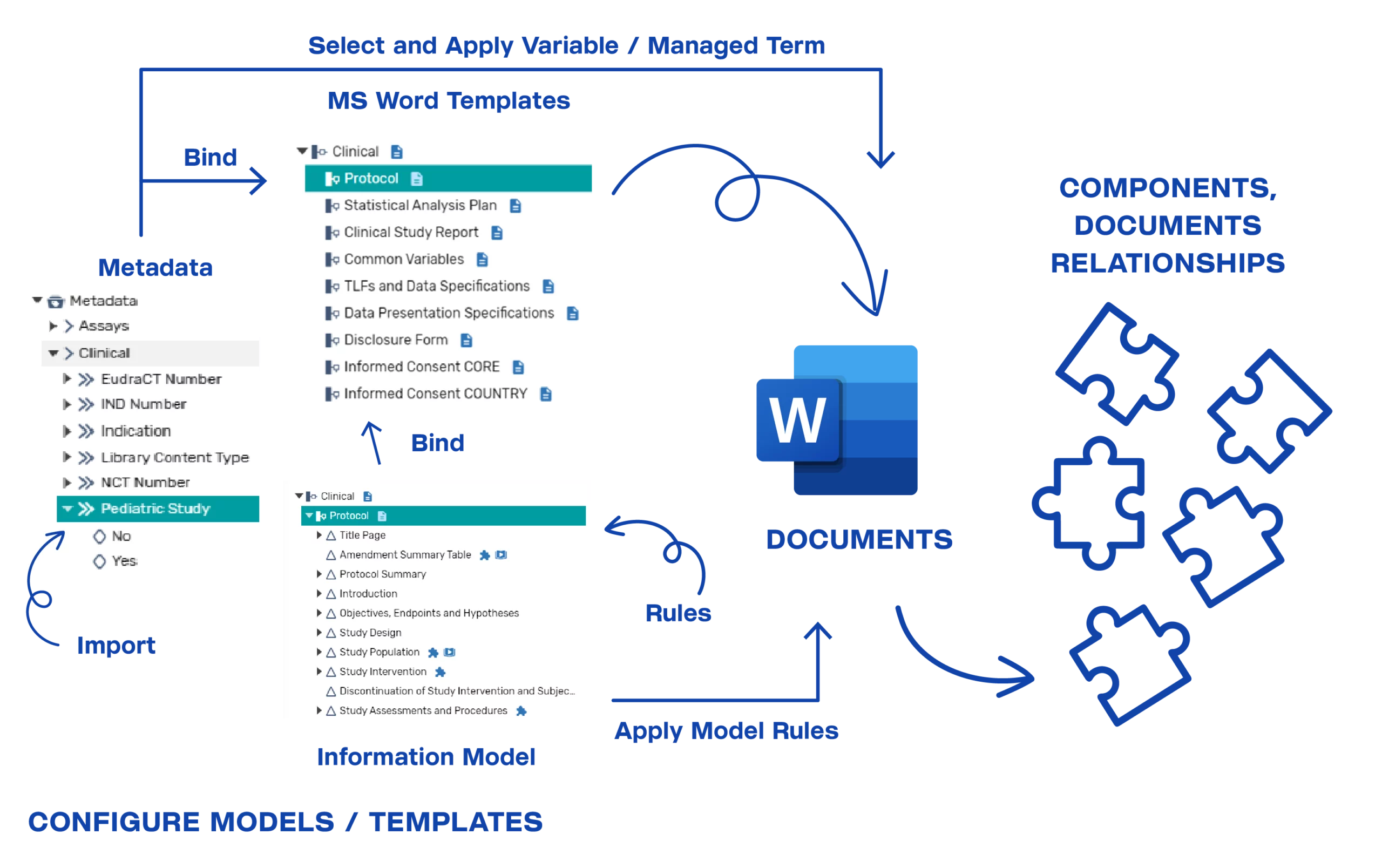
Metadata Model Configurations
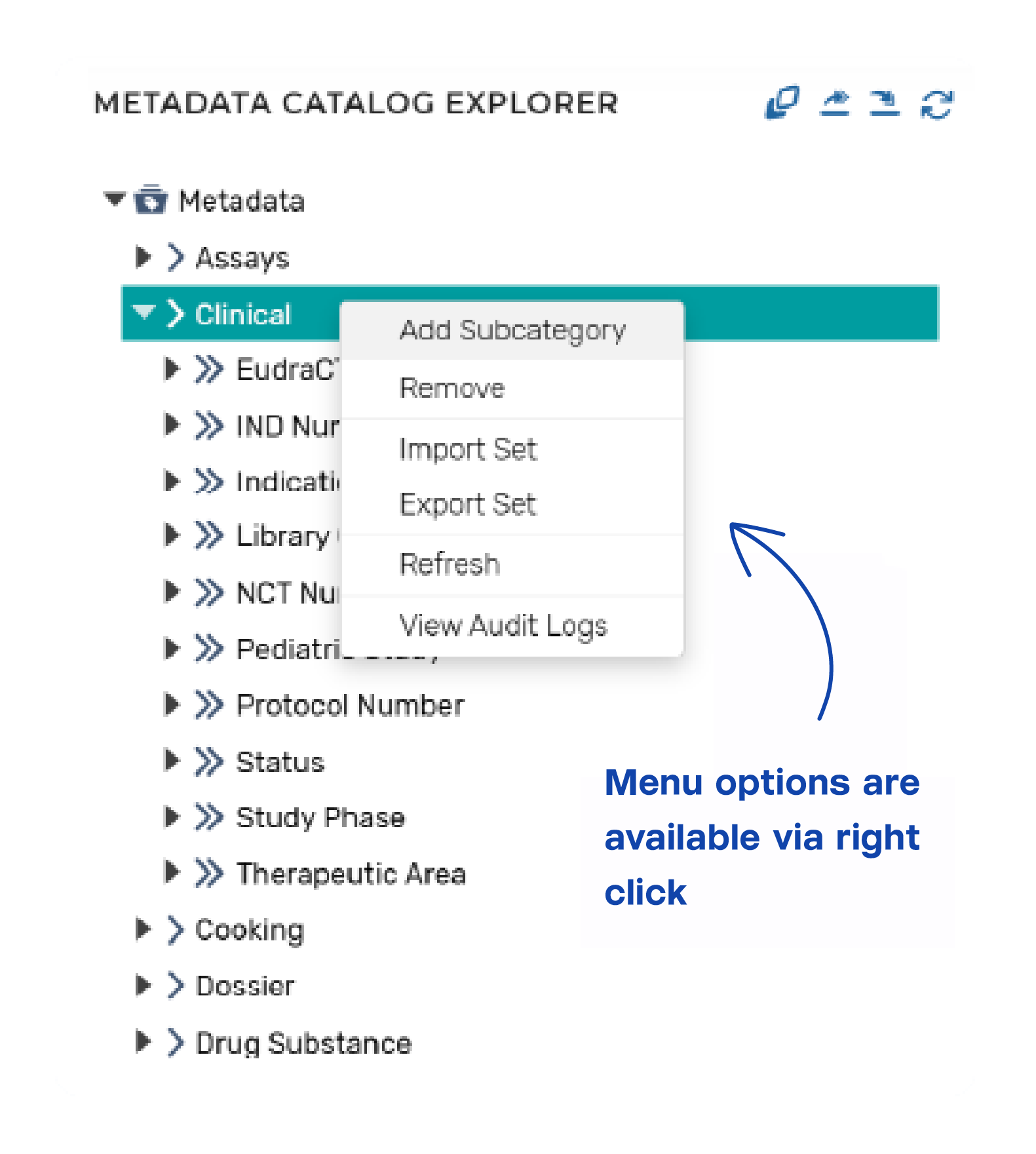
The platform provides the capability to configure and manage metadata models to support business needs. The metadata model is used to classify components and documents managed in the system, to enable auto-population of content within a document or dossier, and to bind document templates so that authors can populate valid term values while authoring the document.
Metadata models can be:
- Configured within the platform.
- Imported from external sources.
Metadata terms and term values can be:
- Sourced from and linked to external data sources or databases.
- Exported for use in downstream systems/needs.
- Configured for related metadata values (e.g., countries related to a region)
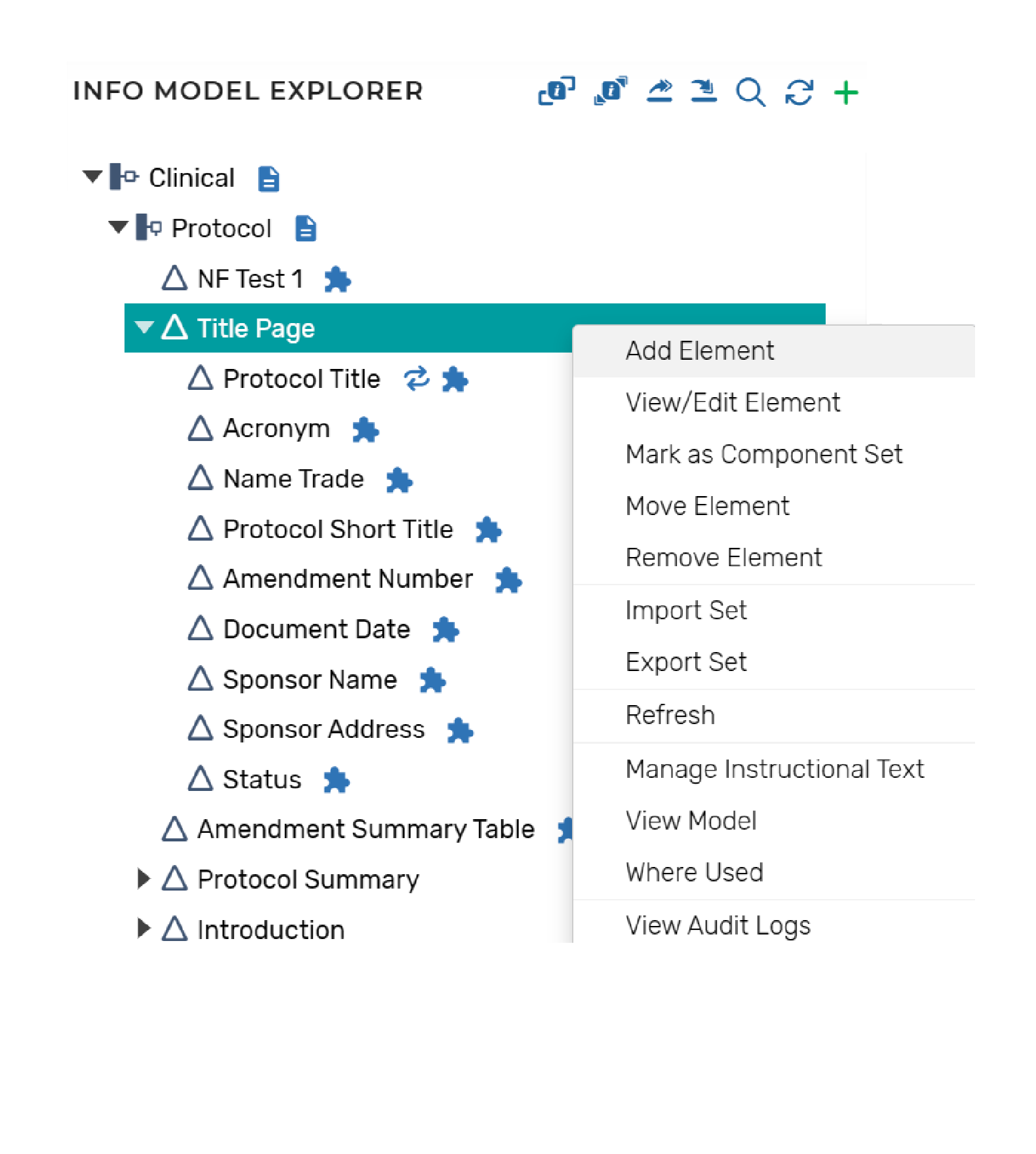
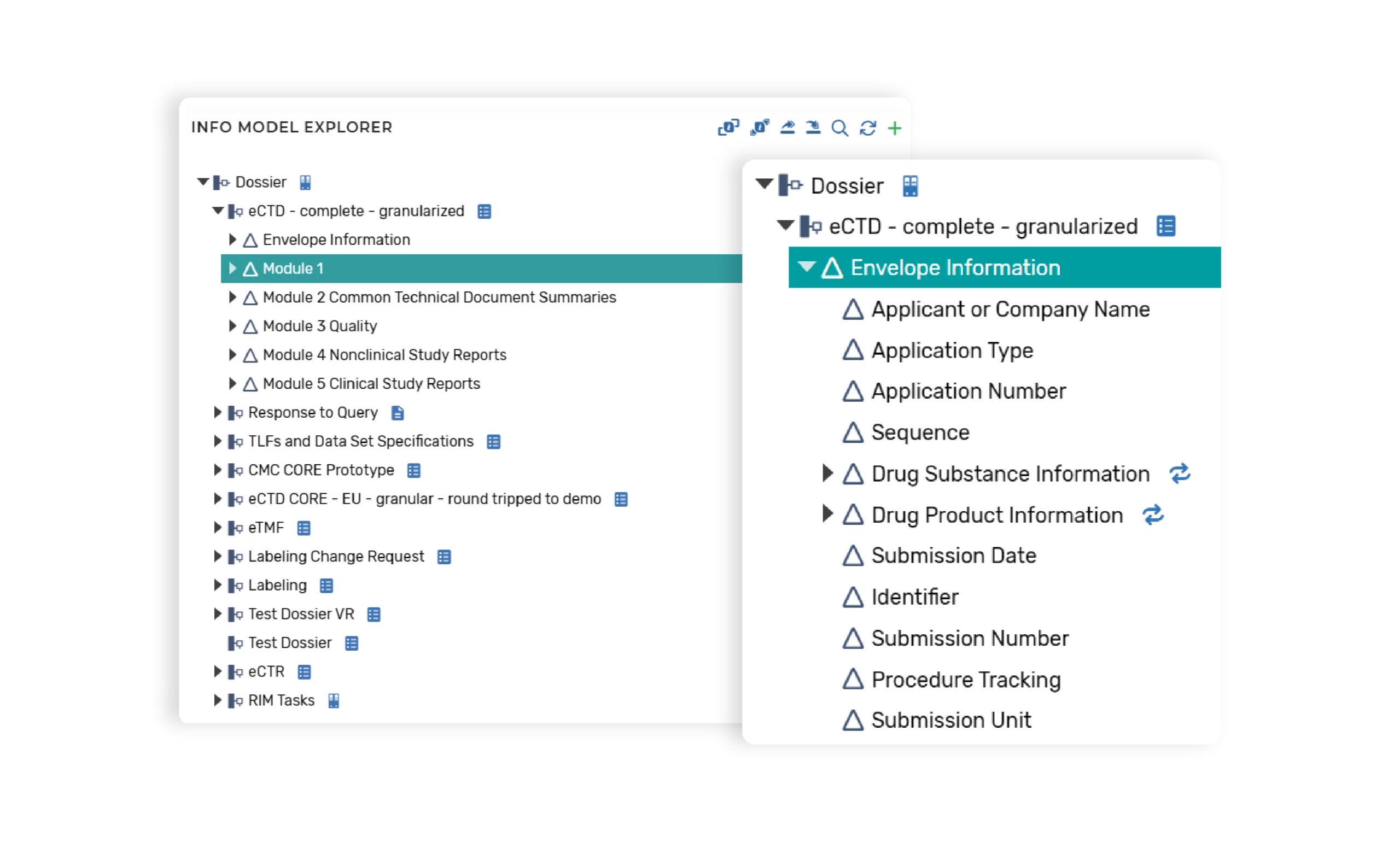
Information Model Configurations
Information models allow permitted users to configure the structure of the document components and define component level content policies, such as:
- Reuse policies and type of reuse (e.g., as-is, repurposed)
- Pre-population policies (e.g., a library where content is to be populated from)
- Variable bindings to the component (e.g., document level metadata values)
- Variable bindings to the metadata model
Information models are configured for each type of structure (e.g., document, dossier) to be supported within the platform.
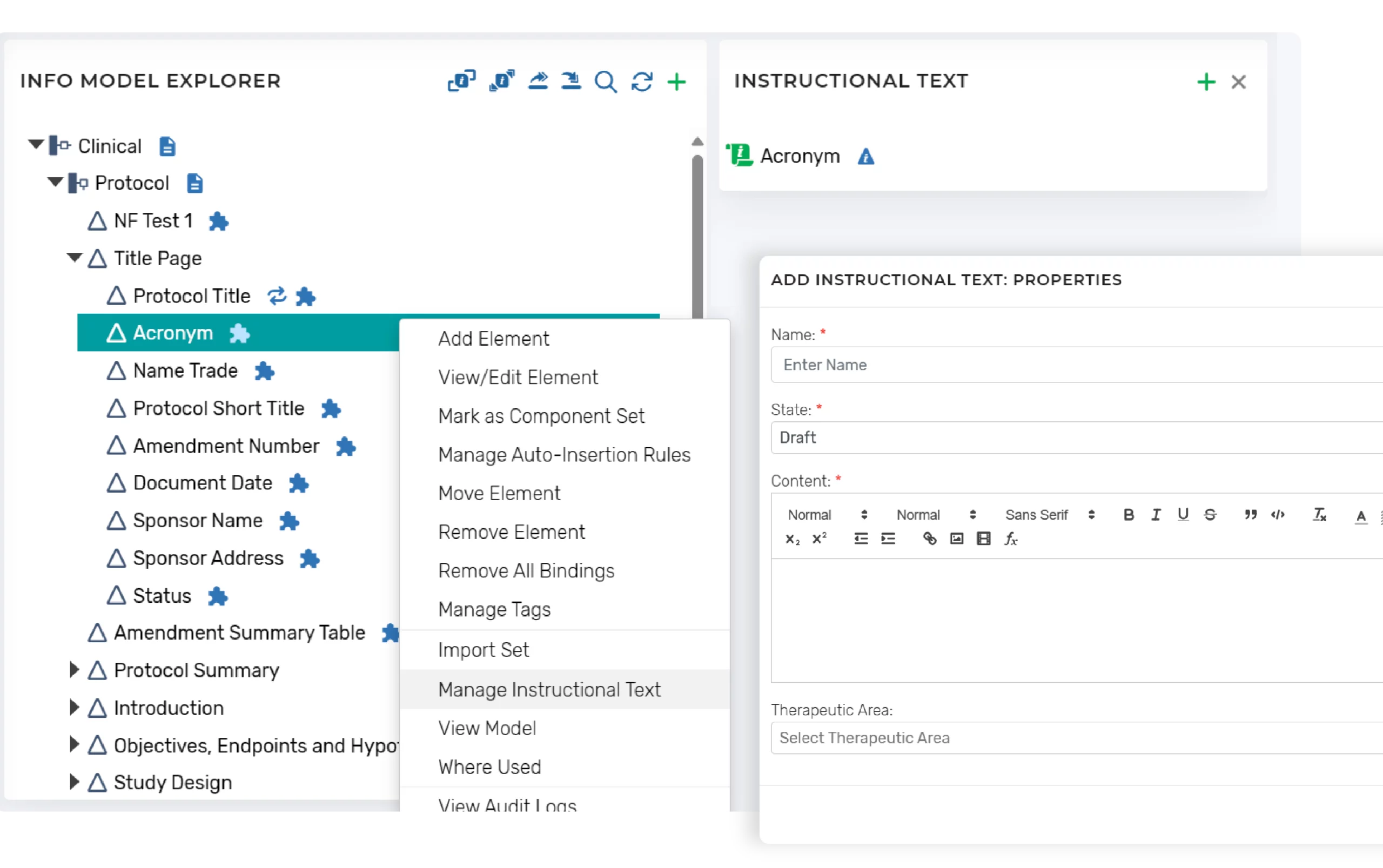
Any related instructional text can also be configured and is kept separate from the document template itself.
Template Configurations
Microsoft Word templates can be configured and bound to the information model and metadata models, as appropriate. The bindings drive how the authoring features are made available to the users. Existing Microsoft Word templates can be analyzed and used to configure the new structured templates within the platform. The goal is to maximize existing templates and work that has been done within the functional areas. The level of granularity of the components is a business decision and based on the level of reuse the business wants to achieve and the maturity/adoptability for structured authoring. The business can adjust and govern the models over time based on their priorities and business objectives.
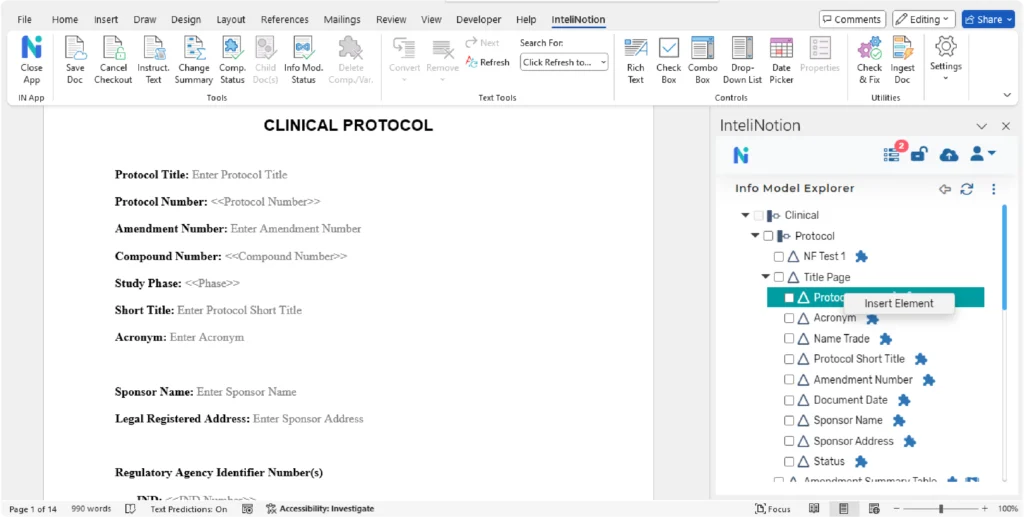
The configuration of the Microsoft Word templates includes some features to minimize the need to revise templates for required updates to instructions or other procedural content (e.g., standard library text, example text) that do not impact the content within the template. This content is separate from the template and presented contextually during authoring.
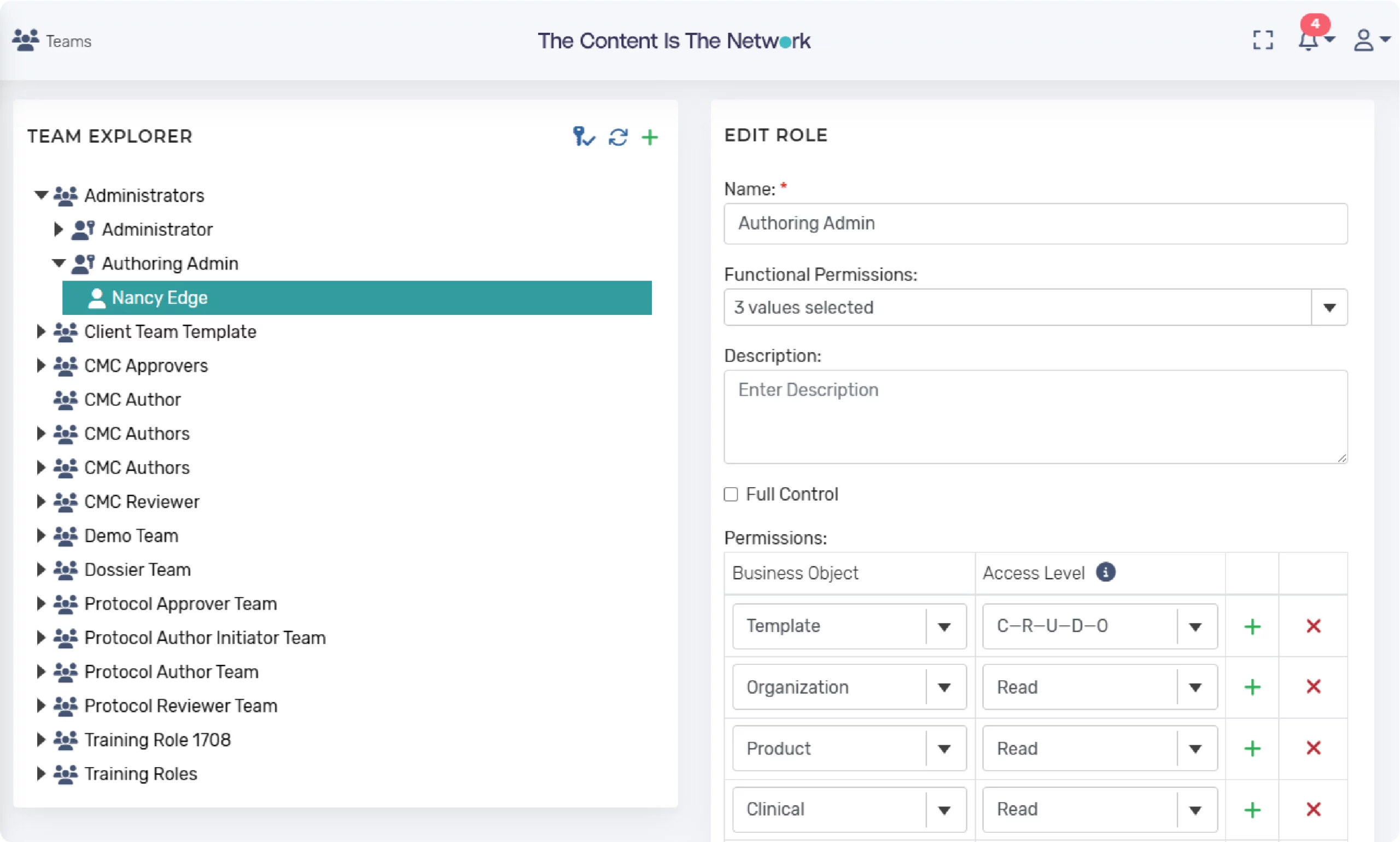
Team, User Roles, and Attribute-Based Access
The platform provides configurability of user roles and creation of teams comprising of one or more roles. Roles or teams can be associated with specific objects to control access. A permitted user can add users to each of the roles within the team. The configurability supports the ability to control access (e.g., for external partners).
Content Library Management
The platform provides capabilities to configure and create content library components that can be created, governed, and made available to authors for use in the authoring process. Multiple libraries can be configured according to the business needs (e.g., standard/required text, example text, therapeutic area specific text). The library components are available for authors to find and insert into their documents during the authoring process.
Document Generation
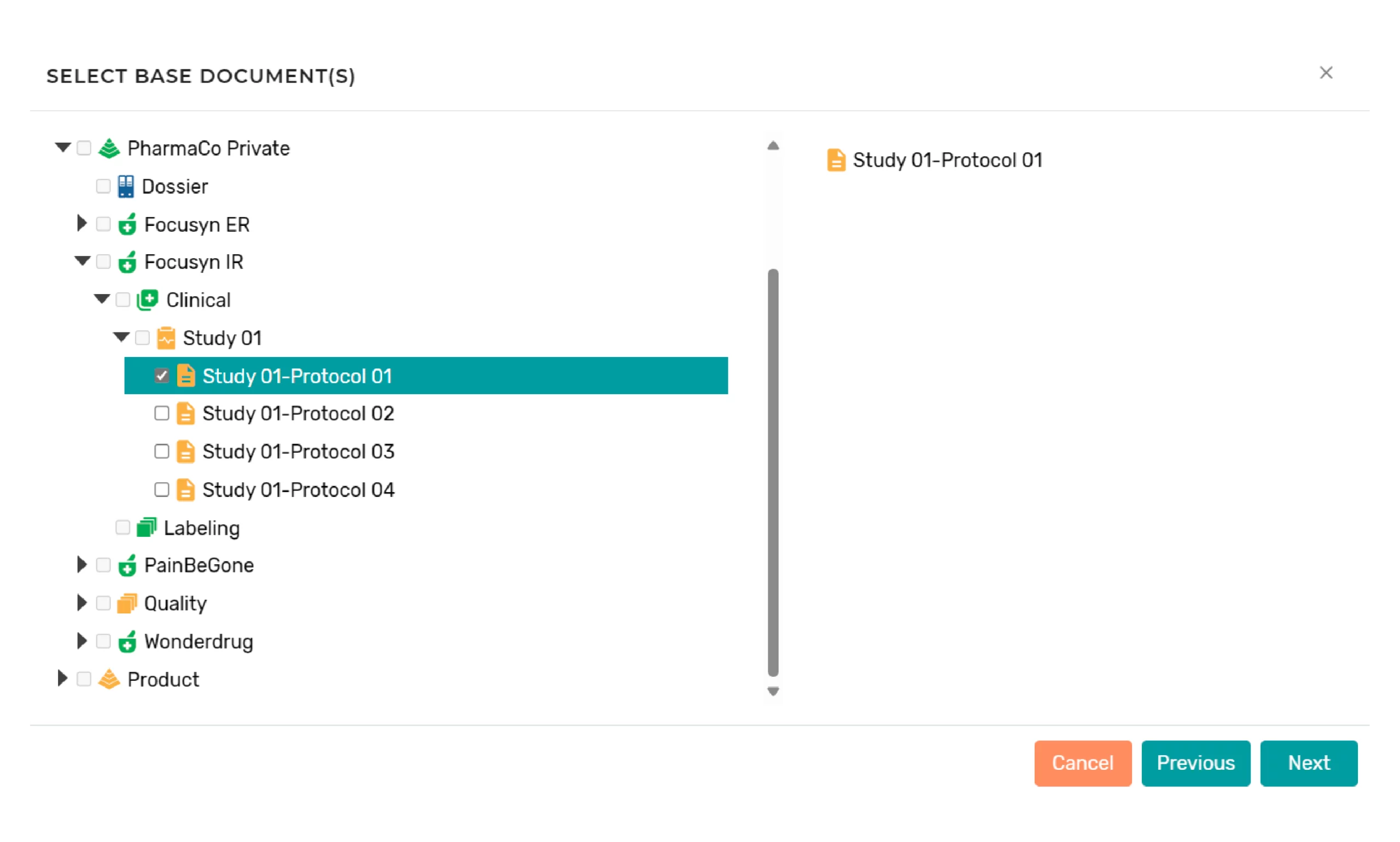
Authorized users can generate documents within the platform by selecting the action and providing the parameters for the document to be generated. When the document is generated, the system will automatically:
- Reuse content from one or more existing documents based on reuse policies configured in the information models; reuse policies determine if content is reused “as-is” (no editing allowed) or repurposed (editing allowed in the new “branch” of the source component)
- For example, a clinical study report body can be generated from reused content from both the protocol and statistical analysis plan, while a company core data sheet can be used to generate country-specific package inserts.
- Auto-populate content from content libraries based on configured rules; rules are prespecified within the information model and are executed based on user-selected metadata at the time of document generation. is reused “as-is” (no editing allowed) or repurposed (editing allowed in the new “branch” of the source component)
- For example, if the user selects oncology for the therapeutic area, the protocol will be auto populated with the Common Terminology Criteria for Adverse Events.
- Prepopulate variables (i.e., metadata values) based on template configurations.
- Maintain traceability of all reused or prepopulated components
- Enable inheritance of metadata from the parent object (e.g., related product- and study-related metadata is inherited by the document)
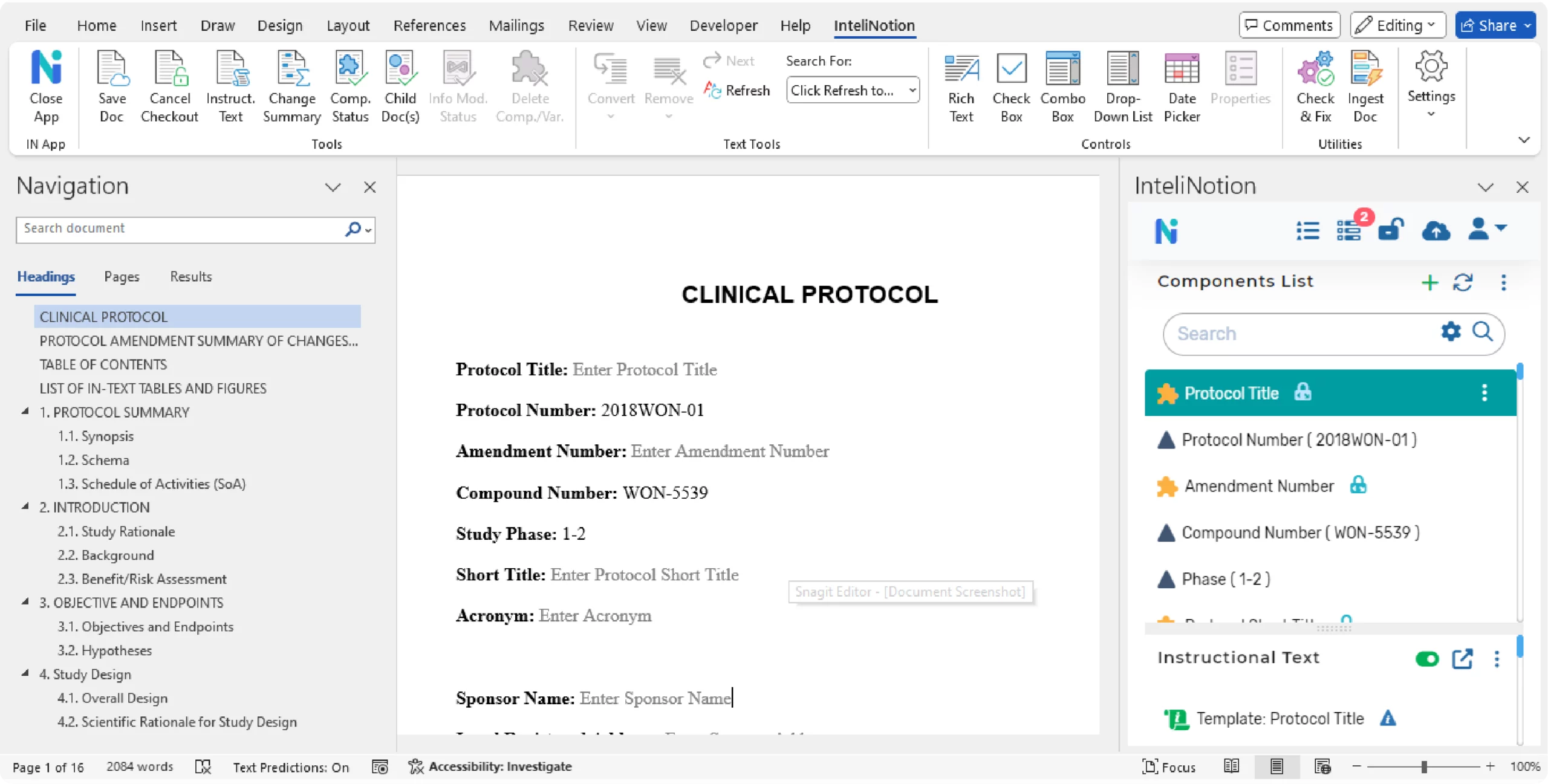
Document Authoring
Authors and reviewers work with the entire document in their familiar user interface, Microsoft Word, via a seamlessly distributed and installed modern App that will be made available from the MS Office Store. The platform manages components transparently by maintaining relationships between a document and its components and by tracking parent-child relationships for reused components. All authoring functionality is available from the Microsoft Word interface. The structured content authoring solution uses the same ICH compliant XML standard used by Microsoft Word, based on the Open XML standard.
Authors will write documents using standard Microsoft Word and be able to easily:
- Insert de novo content (text, tables, images) and apply required formatting and styles.
- Find and insert content from related components previously written for another document.
- Find and insert library content from governed library options (e.g., required/standard text, therapeutic area specific text).
- Insert variables for easy reuse of commonly used and managed terms.
Reuse Reporting
The platform tracks and manages the relationship of the reused components across the entire document lifecycle and the variations or branches of the components. The tracked information is used to provide reuse reporting for parent-child and sibling relationships. Users can view every document where a component is used, the type of reuse, and the metadata related to the component (e.g., version, dates).
Viewing the reuse report is highly useful for users to determine where content is used/reused and to assess impacts of change quickly.
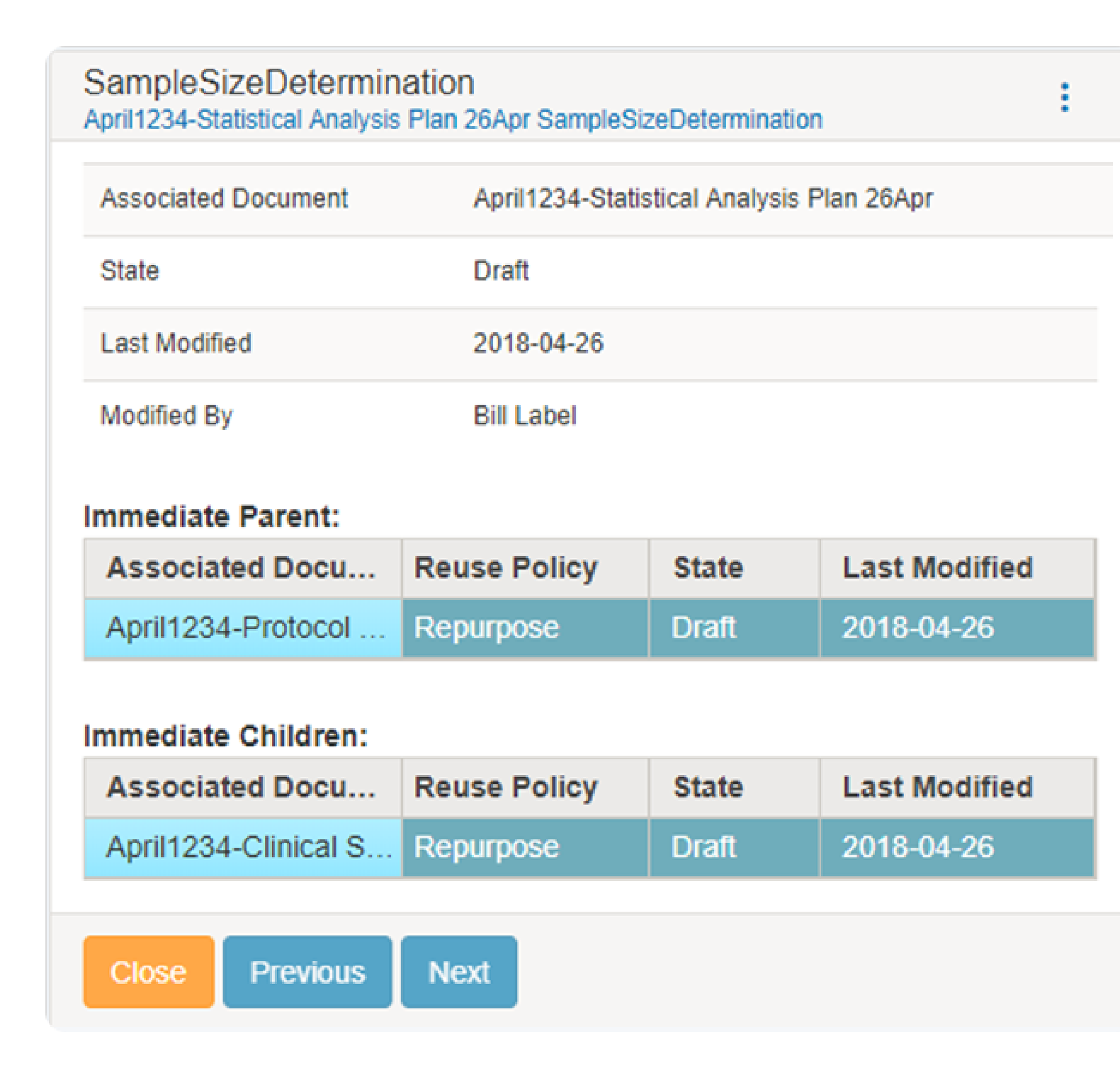
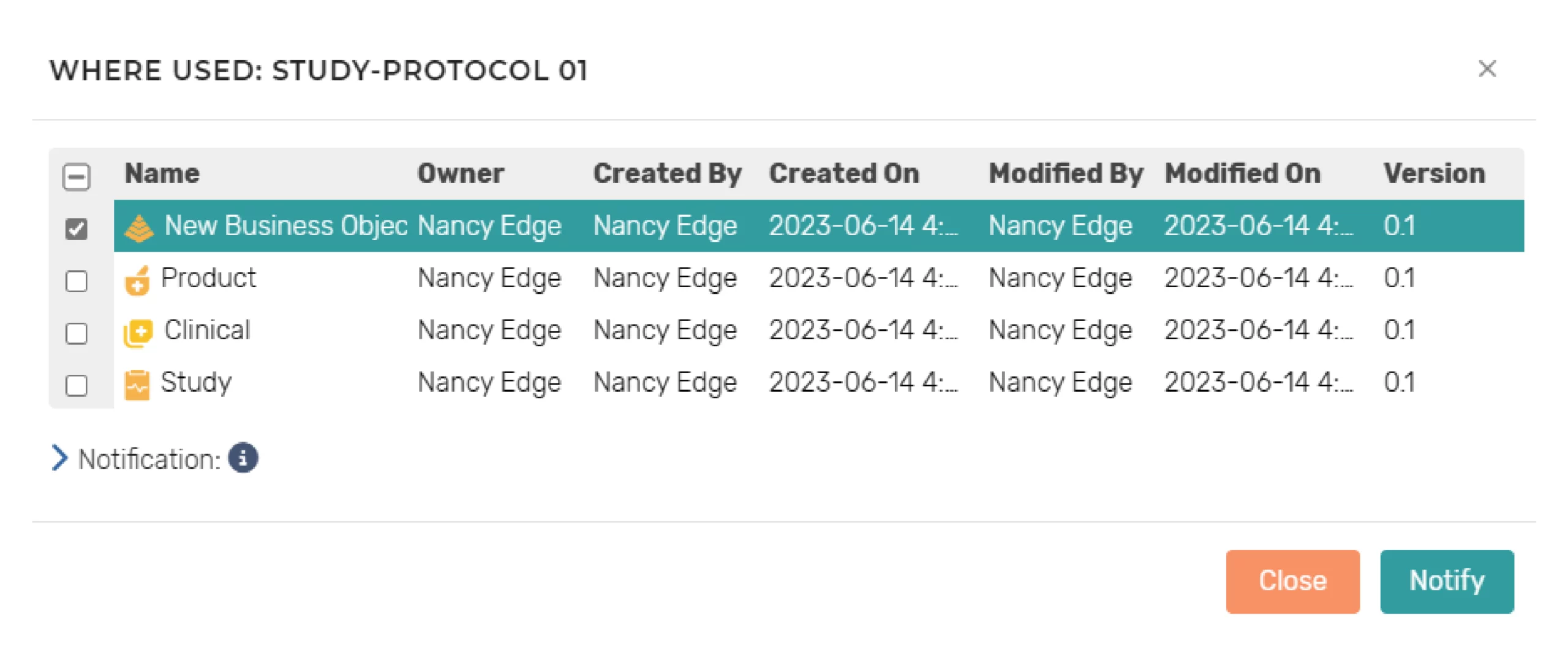
Change Impact Assessment
Users can quickly select a component and view “where used” to determine change impact to the component content. Also, users can quickly select component versions and view differences for change assessment. Additionally, during the authoring process, changes to a component that is reused will auto-generate notifications to lead authors to potentially impacted documents for appropriate action to accept or reject the changes. The notifications are configurable and can also be initiated on an ad hoc basis.
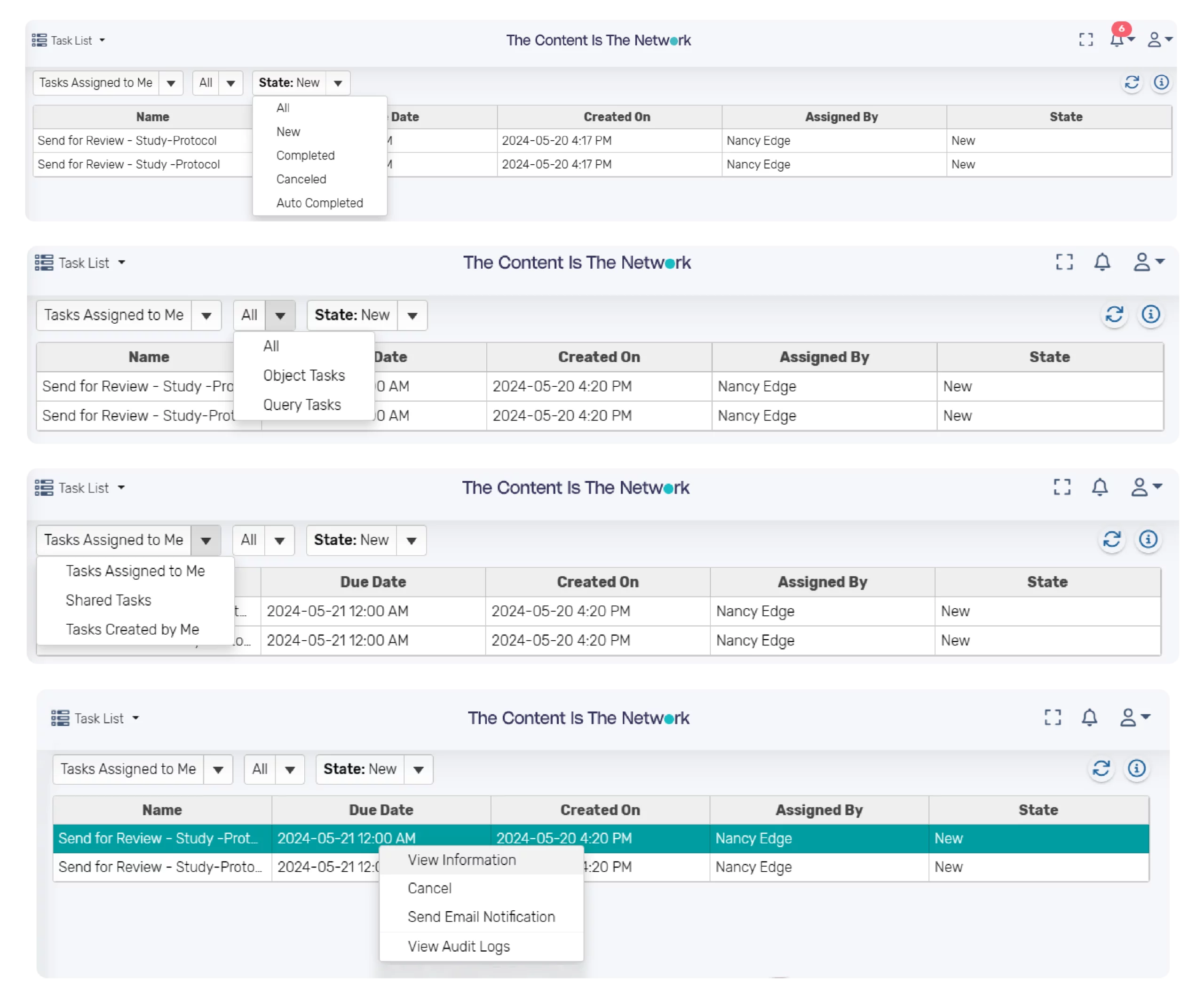
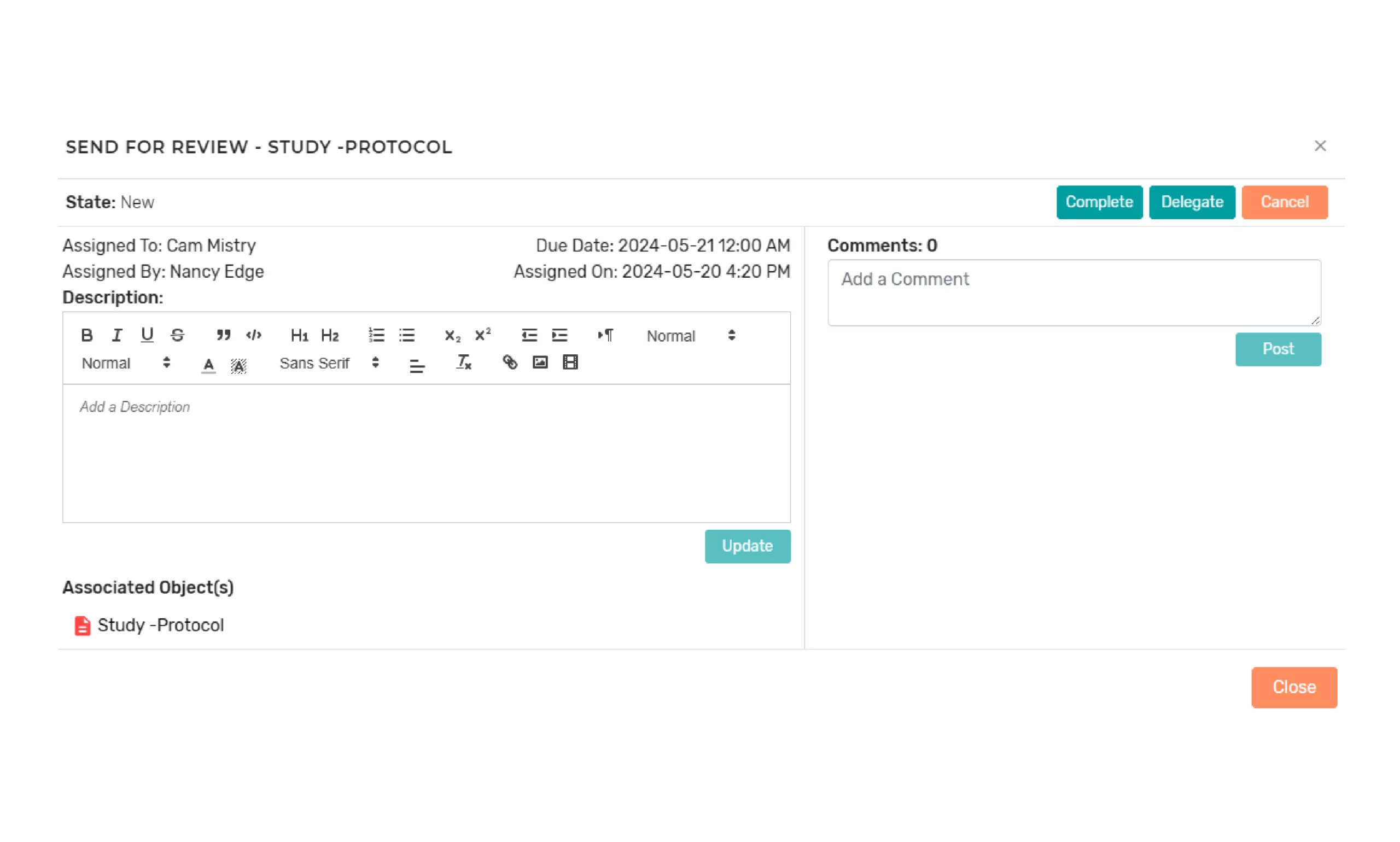
Component/Document Review and Approval Process
The platform provides configurable lifecycles for documents and components and support the routing of components or documents for review and approval. Review and approval tasks are routed to assigned users or teams. If necessary, multiple components can be sent for review or approval together at one time. The task assignee can easily complete a task to finish a review or approval cycle and send a notification, if needed.
Rules-Based Conditional Publishing
The platform supports the capability to preconfigure one or more templates that can be used to generate multiple outputs or variants simultaneously that have a subset of documents from the parent dossier. The templates are configured with rules that specify redaction, deletion, or reuse of the parent dossier components to the variant output. These templates can be associated with specific audiences or regions/countries. When a user selects to generate a variant(s), they create a variant-specific name and the location in the system where the variant(s) is to be created. The platform automatically applies the rules and auto-generates the requested output.
Text-Level Redaction
The platform provides the ability for users to redact content at both the document and component level. A user can highlight specific text for redaction and then show or hide the highlighting during authoring. This makes the redaction process more efficient as it allows the redactions to be made at the time of initial content development. The platform will provide the ability for a user to select what type of redaction to perform: “anonymize” the highlighted content; change it to non-searchable text characters; or remove the content completely.
Connectors to GxP Repositories
The platform is repository agnostic and can provide connectors to backend GxP and other repositories. Documents and components can be stored and managed in the backend repository while the platform provides the productivity tier for structured authoring. The platform will provide connectors to Veeva, Documentum, and SharePoint.
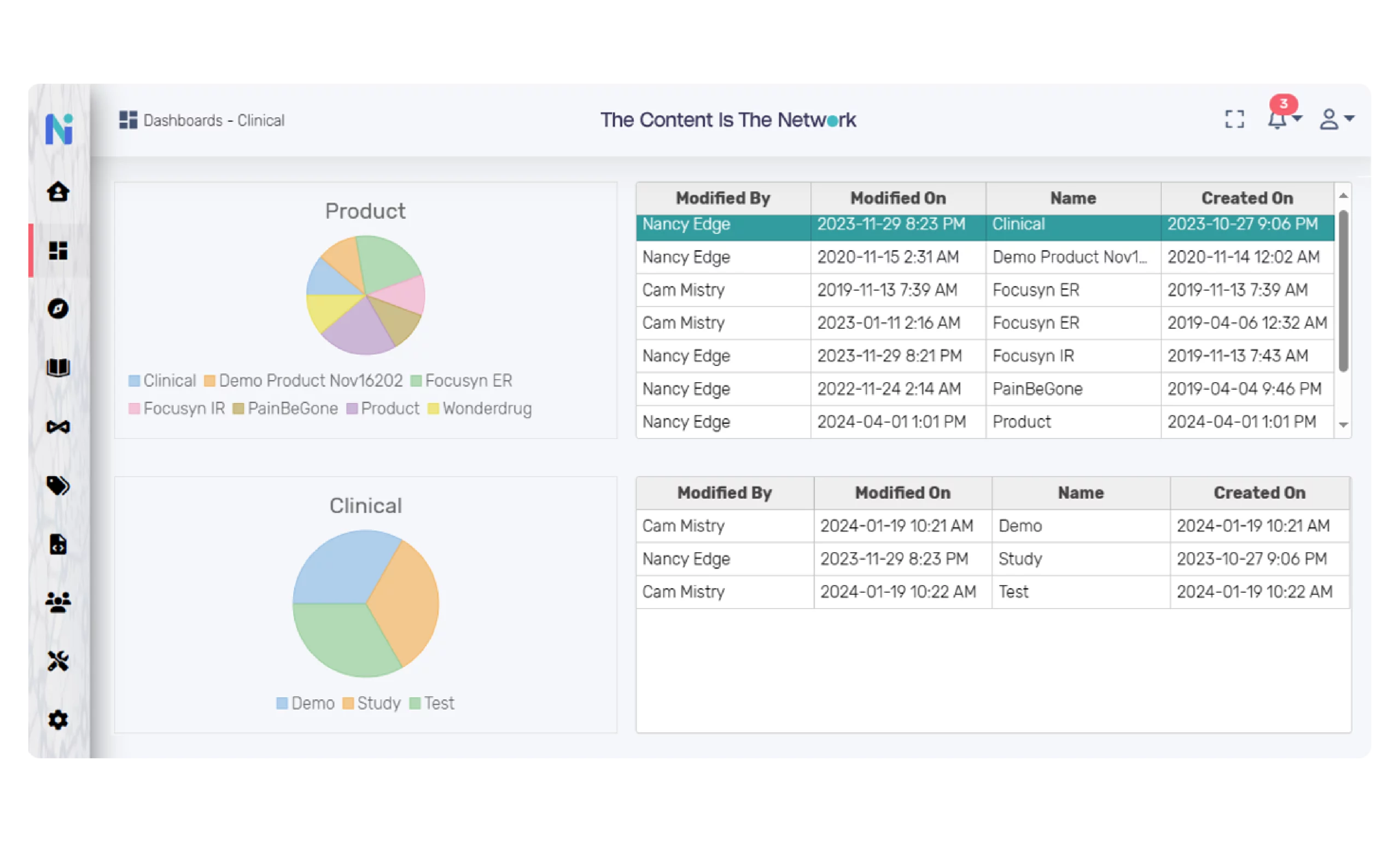
Dashboards and Reporting
The platform provides configurable and actionable dashboards where users can navigate from the higher-level functional area to the documents and related components. For example, users can configure and view dashboards that will allow drill-down from study to the study-related documents, and from a document to the document components. Metadata associated with each level are configurable and actionable. For example, a user can select from the dashboard to view versions, open a document, or edit a document or a component.
Solutions and Delivery Approach
We deliver different modules for various functional areas by leveraging the core platform capabilities and deliver preconfigured base models and templates for key documents and content for the functional area (e.g., clinical, CMC, labelling).
Training documentation on how to review and adjust the models for your needs and rapidly configure the platform to meet your template requirements will also be provided, with the objective of completing a rapid pilot program within three to four months. Additional document templates can be easily added to the solution to support your broader needs.
The features are specialized for the specific roles within the functional teams such as:
- Information architects and standards team members who will adjust the information and metadata catalog models.
- The template management team who, with minimal training, will adjust the Microsoft Word-based document templates and rapidly configure the appropriate bindings to the metadata and information models.
- Authors and reviewers who will use standard Microsoft Word interface for all their needs.
- Document specialists who may assist the authors in setting up the template configurations for the final publishing of the outputs for regulatory repository or other downstream uses of information.
Value Delivered
The InteliNotion platform facilitates a rapid configuration and pilot approach to enabling structured authoring for the documentation within life sciences (e.g., clinical, CMC, labeling), delivered on the trusted Microsoft Azure Cloud platform. Whether your authors and reviewers are within or external to your organization, users can leverage the same set of tools and follow the same processes. Our solution, together with the content expertise our system integration partners deliver, will allow you to implement your processes for structured content authoring directly with your business partners. This shift in the authoring paradigm will improve quality, ensure consistency, and deliver significant value and efficiencies in creating, reviewing, approving, and tracking the hundreds of related documents created through the product development lifecycle. In addition, structuring the content also promotes easier methods for downstream uses of the information that is currently embedded in documents by allowing the appropriate content components to be published/output into required formats for downstream systems and applications.