Solution Brief
Features
Business Challenges
The end-to-end submission management process is very complex, supporting many cross-functional processes such as manufacturing, R&D document authoring and clinical trial submissions. Planning, compiling, submitting and tracking a global submission dossier is a major project undertaking involving all necessary stakeholders – including the medical writing team, document reviewers, approvers, marketing experts, and the regulatory operations team. Often external partners and third-party vendors also participate in the process.
Additionally, minor changes in documents, labels, statistical analyses or other content create a ripple effect through potentially thousands of documents over many years. Today, change impact assessments across global submissions require a manually intensive process and is generally managed using spreadsheets and multiple systems. Multiple repositories and systems manage the end-to-end regulatory processes. These repositories and systems are not able to provide an end-to-end view of global submissions, supported by role-specific views of the information and an integrated audit trail of the end-to-end process. This functionality is necessary in order to achieve transformational process improvements, improve information quality and consistency, and comply with FDA 21 CFR Part 11 for electronic records.
Regulatory professionals need visibility into critical information, which is too often isolated in disparate systems and document repository silos, negatively affecting regulatory and operational compliance, strategic planning, timelines and productivity. Without a holistic view of regulatory information management as an enterprise-wide process, the organization will continue to be hampered.
Using structured content management principles combined with an end-to-end Information Management platform enables delivering a transformative, regulatory information management solution to track and manage complex global regulatory information and processes, including correspondence, commitments, risk assessments, approvals and other related submissions artifacts. Such a solution can help regulatory professionals better plan, coordinate and execute their regulatory activities and achieve continuous end-to-end compliance.
The InteliNotion Solution
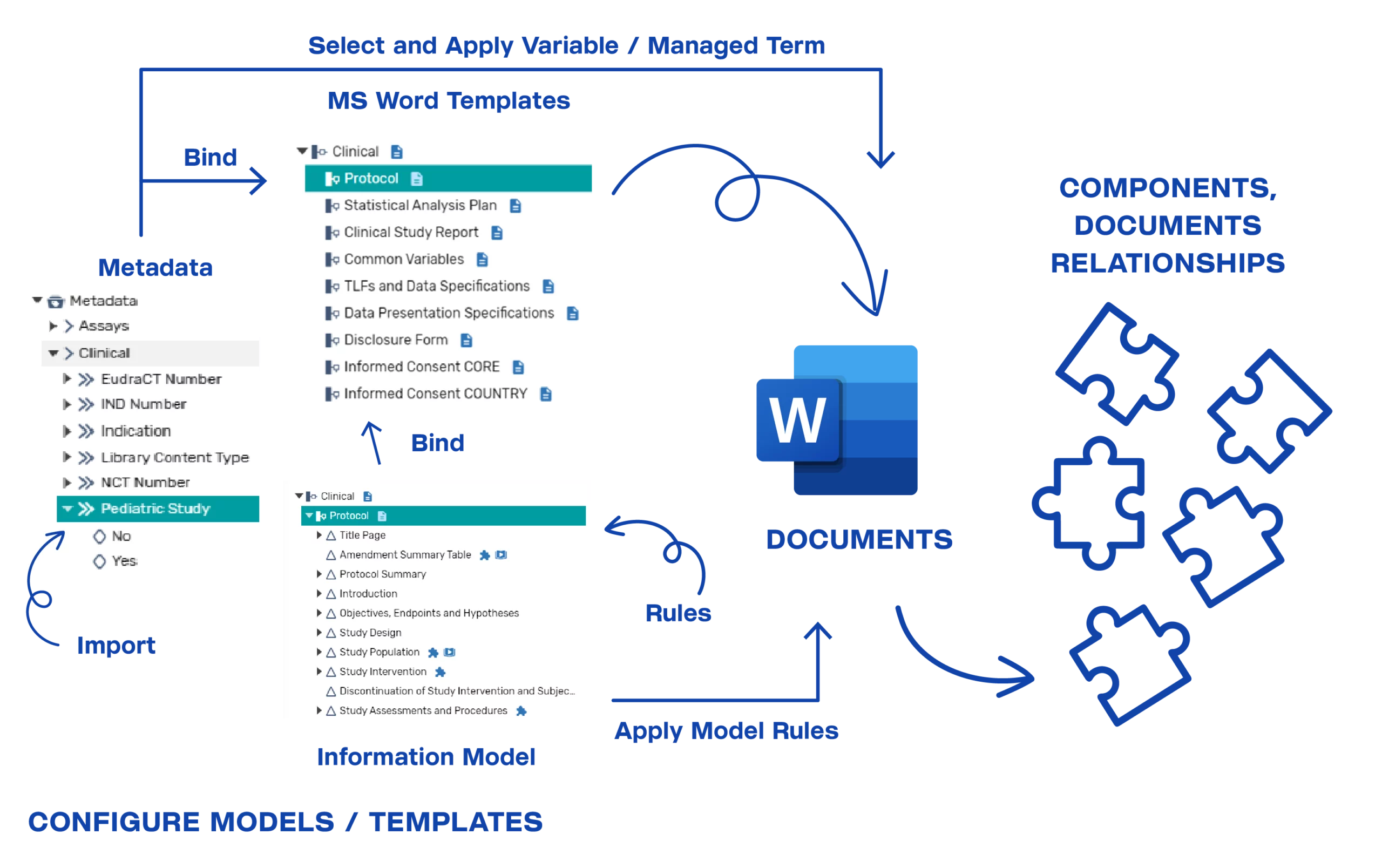
InteliNotion delivers a patented, revolutionary Structured Content Authoring, Component Content Management and Content Governance Cloud native SaaS solution built on the latest Web technologies that provides a set of unique business capabilities, introducing a sophisticated yet user friendly approach to Dossier Management. The platform, together with specific pre-configured models, templates and rules, provides a rapid configure/deployment approach to support regulatory submission management and tracking needs. Our solution supports key capabilities of the submissions end-to-end process for structured content management, structured content authoring and dossier management, with appropriate integrations to our clients’ GxP repository, submissions program planning and publishing systems, as depicted in the following diagram:
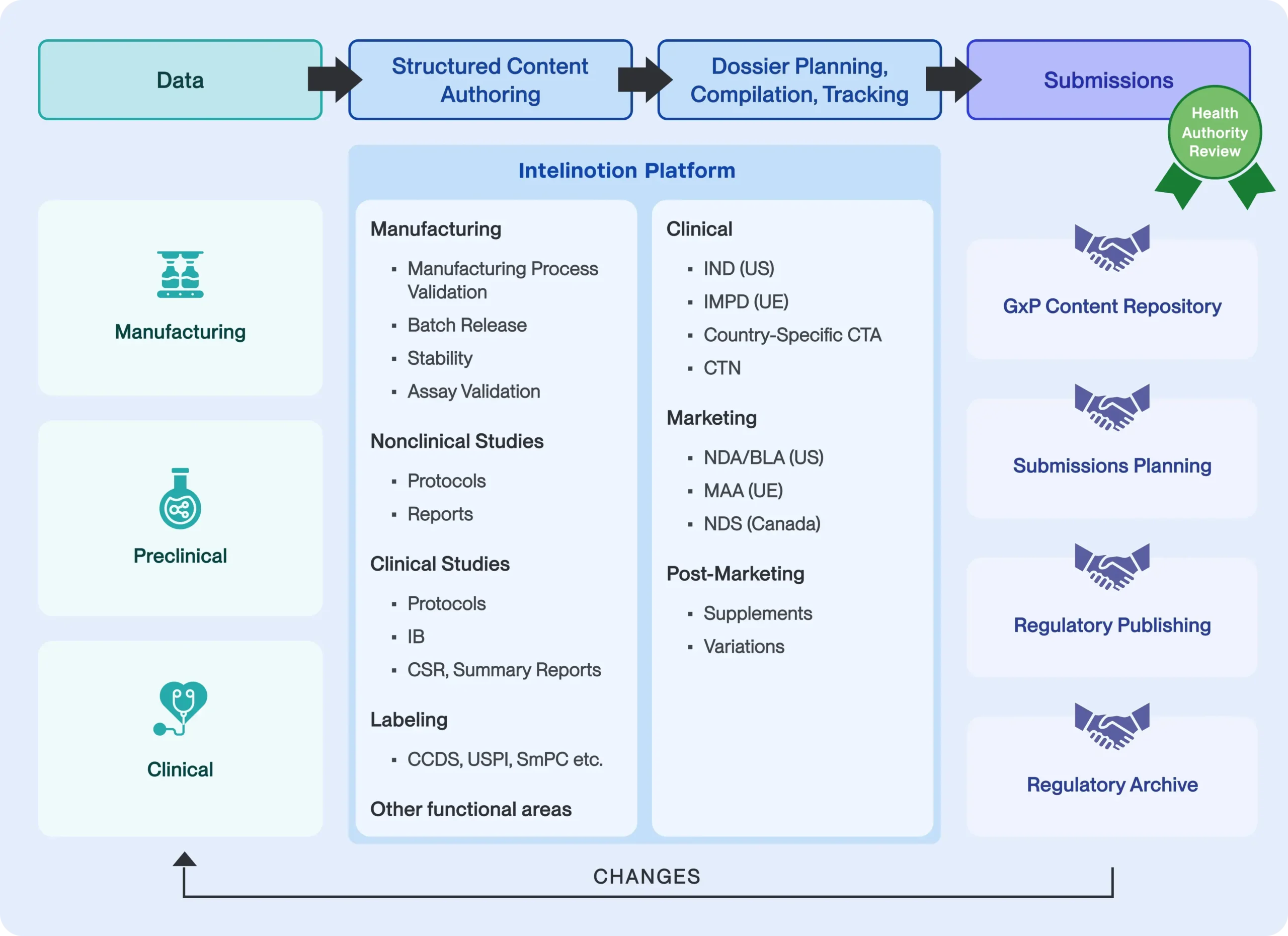
The following are some of the key scenarios addressed by our solution platform:
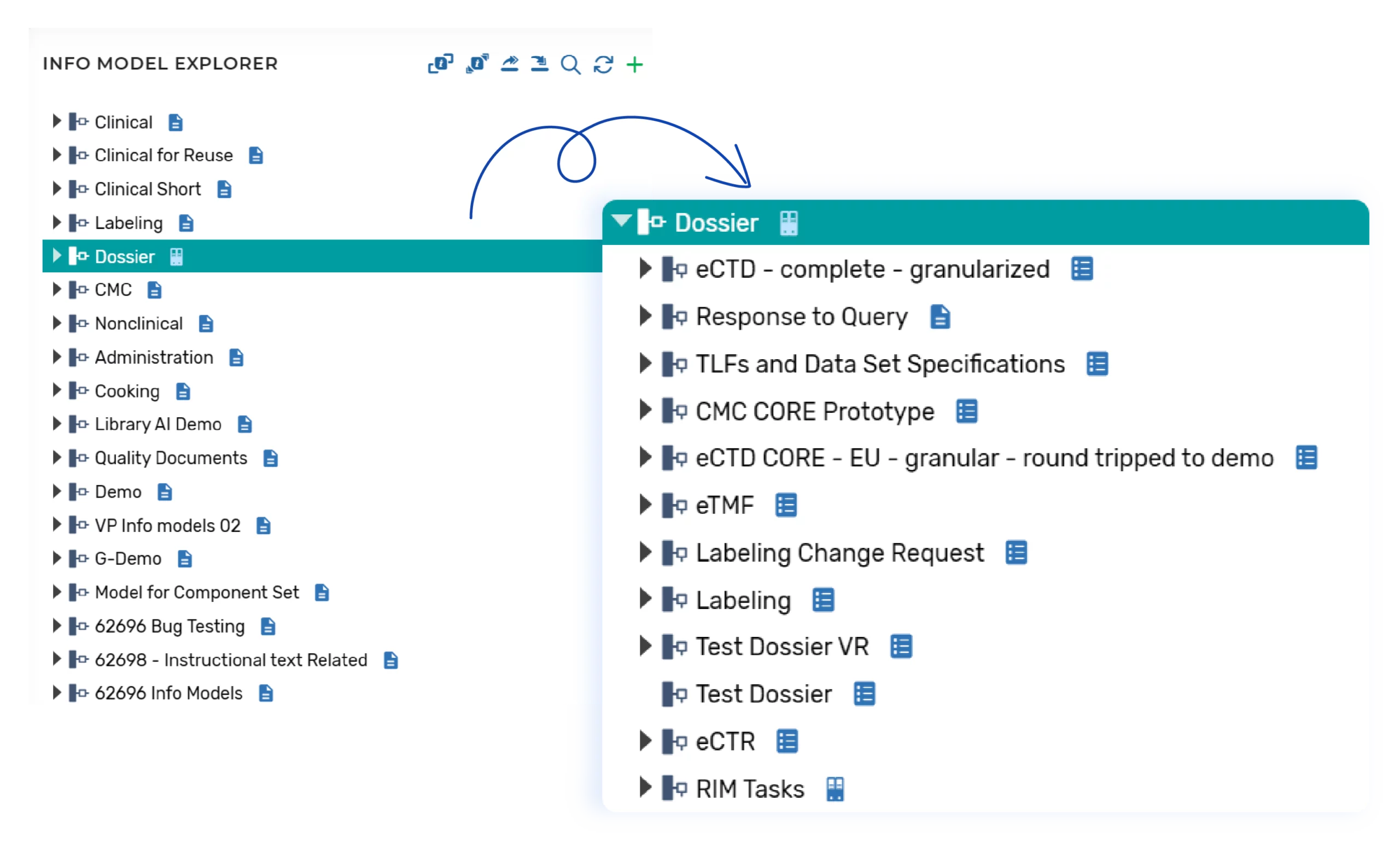
Create Dossier Plans
Submission Planning is initiated by the Submission Manager within the InteliNotion productivity platform. Plans can be created from previously configured templates using a form where the Submission Manager will select the applicable dossier templates, and populate the submission metadata for the plan:
- The structure for the plan is created from the template for the related components of the dossier
- and the relevant metadata is auto-populated into the structure
- The information model configurations within the selected template are used to create the structures, role-based security permissions and relationships when creating the plan.
- Rules can be configured and associated with the templates and are applied when creating the plan (rules related to security controls, notifications, and alerts etc.)
- Plans can be extended to add additional information to the plan:
- Submission Managers will be able to extend existing plans at any level.
- Rules can be configured and applied that control when and how the plan can be extended, and applied when users are making the extensions.
- The system will be able to track the variations/extensions to the plan from the original template or existing submission structure.
The following user interface illustrates how the Information Model drives the dossier structure when a dossier plan is created and metadata population for the new plan:
InteliNotion treats a dossier structure and related components in the same manner as components within a document – a structure of components with related policies for each component. Our solution provides the ability to configure information models with “reuse” policies that can be pre-configured across submission dossier structures. This supports a highly flexible and adaptable approach to manage reuse across global submissions. When content is populated into the parent structure, the ‘reuse’ configuration and policy will automatically relate the content to the relevant global, regional/ country specific submission structures, and track where it is reused. As the reuse policies are dynamically configurable by the business functions such as an information/ data architect or regulatory specialist, the flexibility of the solution is significantly enhanced.
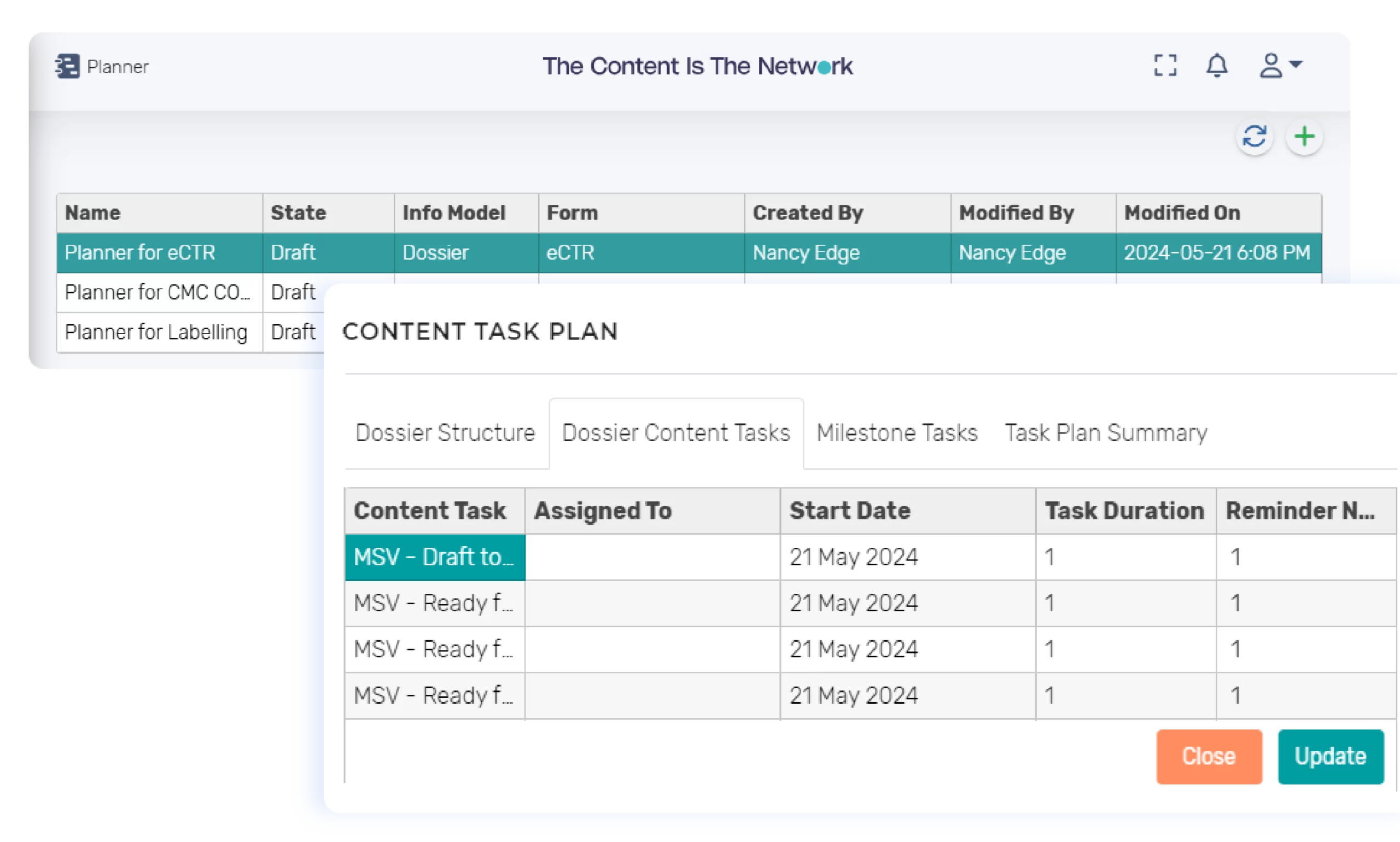
Dossier Project Planning and Tracking
The InteliNotion solution facilitates the generation of a dossier project plan from the dossier structure. Pre-configured tasks can be configured for components within the dossier – example: Compile, Review, Approve. Roles can be assigned to the tasks. Tasks can be added for milestones such as Review Meetings, etc.
The project manager can initiate and assign tasks with end dates. The platform will route tasks to the appropriate roles for completion. Our solution provides the capability to track status of the project plan to have visibility to status of all tasks for actioning. Notifications are sent to the task owner and initiator (configurable) when task deadlines are approaching or missed.

Document Upload / Bulk Load
Content can be imported into the dossier structure either as individual files or in bulk from external content sources:
- Individual files can be imported and associated with the dossier.
- The information model configurations within the dossier structure are used to auto populate the applicable metadata for the content
- Rules configured and associated with the information model for the dossier structure are applied
Bulk import of content is supported as follows:
- A configurable mapping structure can be used for the bulk import of external content.
- The source content for the bulk import can be uploaded and the mapping configurations used to organize the imported content into the submission structure, and auto populate applicable metadata. Applicable rules configured in the information model are applied at the time content is imported and stored.
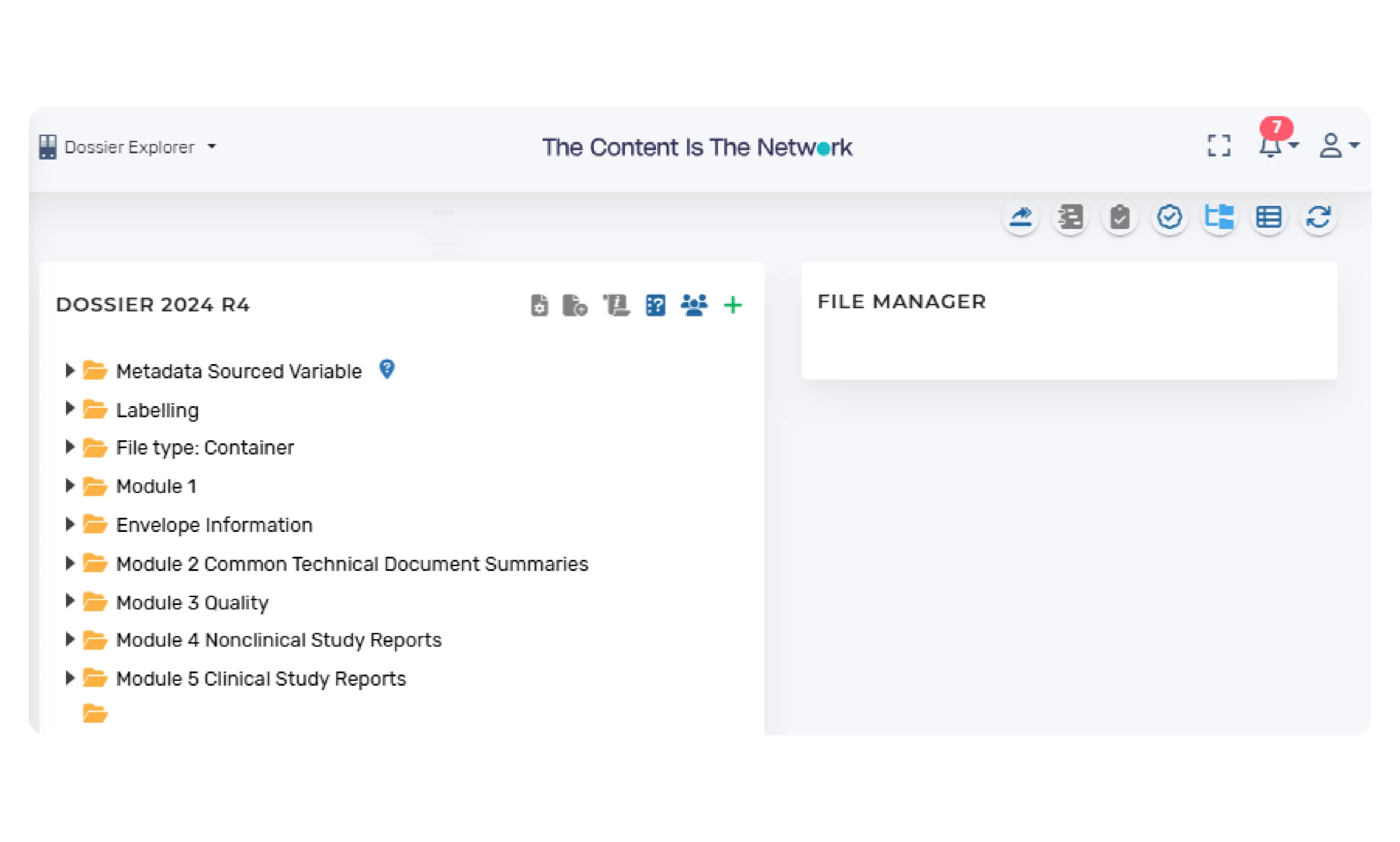
A dashboard view is available with visual icons to easily view the imported content and status of the entire dossier.
Populate Dossier Structure
Linking content to submission structure:
- Authorized global users can link content to the submission dossier structure. The status of each of the elements of the structure are maintained (i.e. Not Populated; Draft; In Review; In Approval; Approved etc.). These statuses are used to visually provide regulatory stakeholders and users with easy visibility in the overall status of the global submissions.
- Content can be linked as appropriate for the core submissions as well as the regional/ country specific content. When configuring the dossier plan for the core and country specific submissions, rules can be configured to determine the user group responsible for the content. Alert notification rules can be configured to send alters to the appropriate user group to remind them of pending need to upload/ link submission content.
Country Specific Submissions Dossiers:
- Local users can use the same functionality and user interface to link or upload local submission content such as administrative documents, translations, other country specific documents, as well as a link to the applicable Dossier components.
Structured Content Authoring
Intelligent MS Word document templates can be created and configured within the platform for use by the authors when creating content. The intelligent templates can be bound to an information model and metadata catalog values to enable authors to select and populate content within the document during the authoring process. Based on the template bindings to the information model and metadata catalog elements, when the author creates a new document within a submission structure, component content and metadata can be auto-populated as appropriate.
The following diagram illustrates the information and content model binding to Word templates, and the authoring process flow:
Rules can be configured and applied that control document status and role-based security for the lifecycle state.
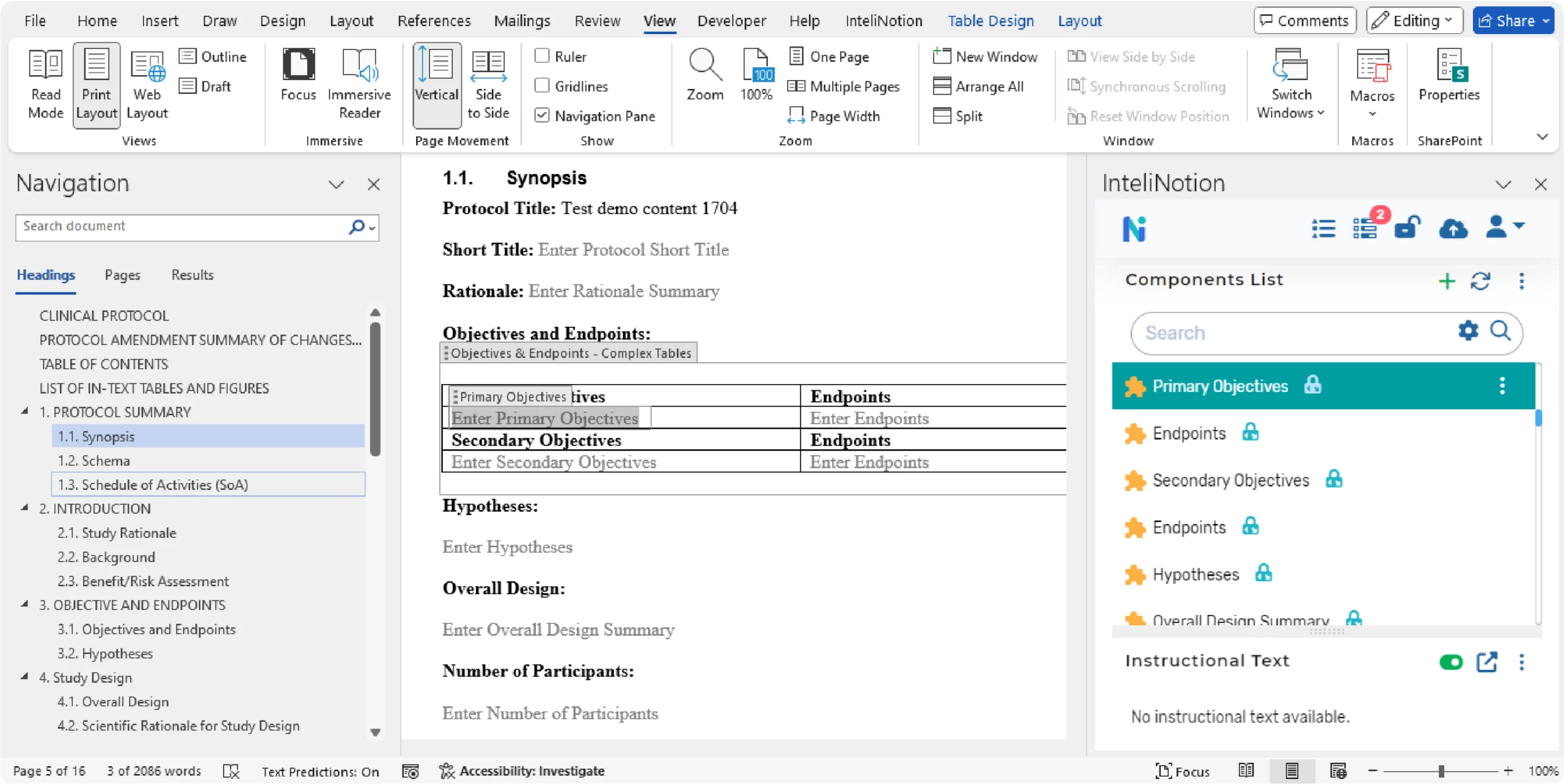
Users can tag content within the documents that can be used to remove and publish an audience specifics output containing only the content that is applicable for the target needs. Content tagging is supported through the authoring tool in MS Word as illustrated in the following user interface:
Translation Management
Content from external sources such as a Translation Portal can be linked within the dossier structure or uploaded so that the content is managed/ stored within the repository. Translated content is related to the source content from which the translation was completed, and full traceability of the original content version and the translated content is maintained. If the original content is changed, alerts can be generated to notify appropriate users to review and take necessary action for the translated content.
Information Findability/Search
Our solution provides innovative approaches for users to access information, such as:
- Actionable role-based dashboards provide transparency of regulatory content and activities that present information to users which they can drill-down into for detailed information.
- Actionable dashboards can provide transparency on the status of regulatory activities and intuitive information access for the entire drug portfolio.
- User-specific dashboards track the relationships between relevant correspondences, commitments, and submissions all in an integrated view. Users will be able to access and view the information needed to improve visibility and productivity.
- Actionable graphical views of end-to-end related information, such as a Dossier plan or a global label submission, which users can view and drill-down into for information.
- ‘Google-like’ search to find information using metadata.
- A navigational paradigm in the event this familiar approach is preferred for some information access needs.
Review/Approval Workflows
Our solution provides review/ approval workflows that can be pre-configured for routing documents or related sets of content for review and approvals. Links to the content to be reviewed or approved are included with the workflow task. Users can configure workflow rules that can automate the process of rendering documents to other formats such as PDF, based upon life cycle state, so that once the appropriate document life cycle state is reached, the system will automatically render and save as a PDF rendition of the content. The PDF rendition is immutably related to the original Word document and version.
Managing HA Communications and Artifacts
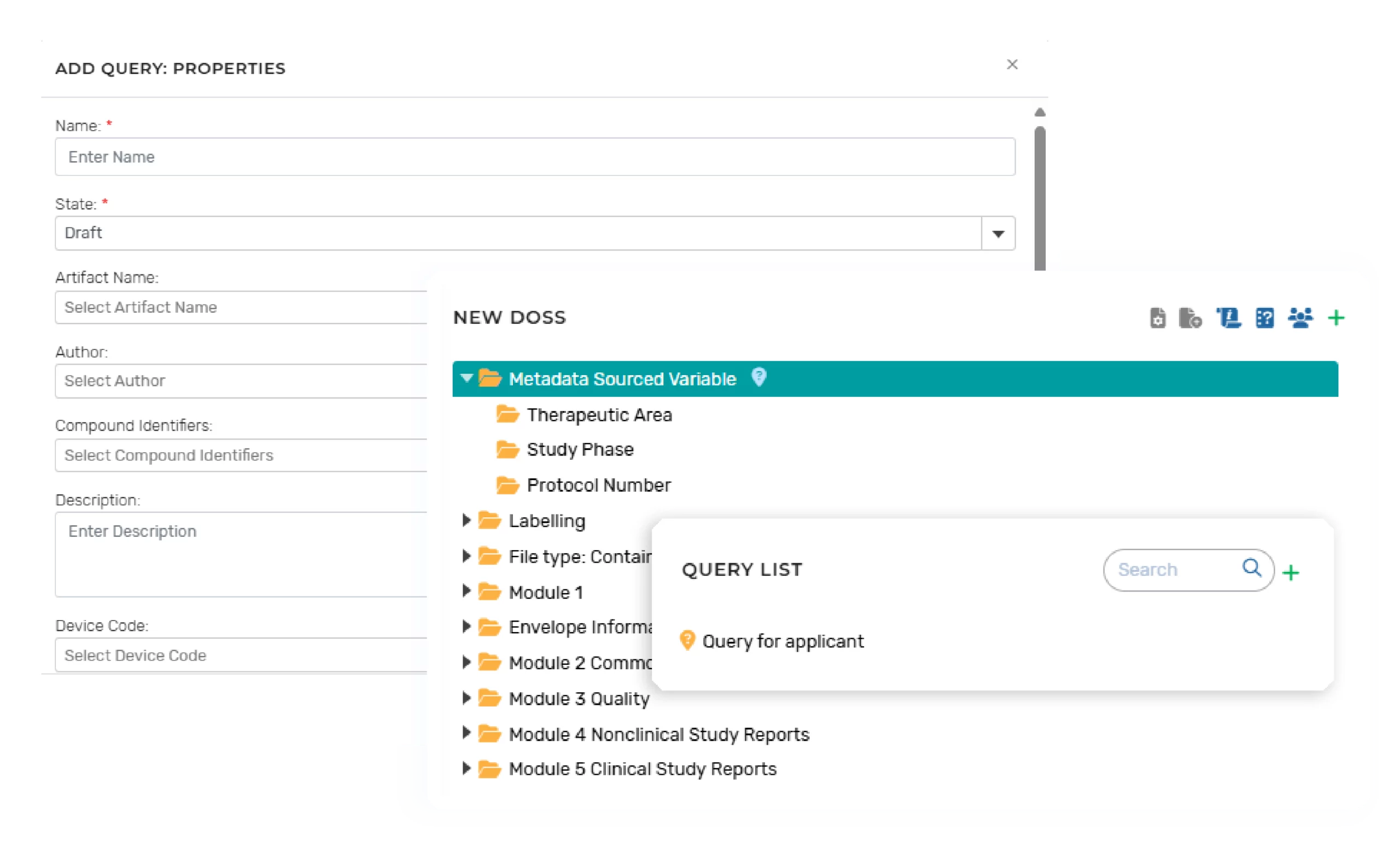
Our solution is designed to support users with the functionality to approve, upload or link appropriate submission artifacts and Health Authority (HA) communications related content. When the content is uploaded for storage, appropriate metadata and role-based security permissions are auto-populated.
User interface example of HA Query and communications related content upload:
Change Impact Assessment
Our solution maintains full traceability and trackability of components across core and country specific dossier structures, variants and branches. This information is used to provide full global visibility of all regulatory information. Reports can be generated that provide the impact of change on any component that affect related components across the global submission dossiers. The reports will also highlight localization variations and translations related to the impacted components across all regions/countries. These reports can be used to develop detailed plans for the implementation, tracking and final compilation of the change.
The reports we provide can be used to navigate global content network as the platform maintains traceability and relationships across the content components. Regulatory and functional users can assess the impacts of change through our ability to make the global traceability of components visible and navigable.
Health Authority Information Management
Our approach is to work closely with our clients to define a cloud-based Health Authority Information Management (HAIM) service that can be accessed using a Web Service. Our team works closely with the HAIM service provider to implement client specific needs.
Change Variation Management
Changes to global dossiers is supported by creating a variant plan from the parent submission structures:
- When creating the variant plan, the user will be presented with a form to compile the metadata associated with the variant plan.
- The variant plan will be created using the existing structure and the content components of the variant plan will retain the links to the parent.
- The Submission Manager will be able to select an existing dossier from which the variant plan being created, as well as select the components of the parent dossier to retain in the variant.
- The platform will maintain the relationship and traceability.
Tasks can be associated with the components impacted by the changes to be implemented. These tasks are routed to the appropriate users/user group for the applicable components for action. Notifications can be configured and routed to alert users of deadlines, life cycle changes, completed activity etc.
Our solution can also import tasks and milestones from external program/ project planning systems and provide regulatory operations the facility to map the imported tasks to components within the global dossiers.
Integrations
Integrations to external systems can be implemented via Web Services, using our modern RESTful API. InteliNotion can be integrated to client systems, such as publishing or planning tools, to automatically push content from the repository, or pull information from external sources. For the end-to-end submissions process, our solution approach will integrate to:
- The client’s GxP regulatory content repository to maintain all documents authored within our platform. Typically, all documents can be generated, authored, reviewed, and finalized within the InteliNotion platform. The final documents can be managed within the GxP repository for approval and used for downstream needs as appropriate.
- The client’s Submissions Planning system – this is the program level planning for the submissions. We can optionally integrate to access all the metadata related to the program and leverage the metadata for the dossier plans.
- The client’s Submissions Publishing system – we can package and generate an out of the Dossier to be published that can be picked up by the publishing system to complete the publishing process.
- Master data sources – to leverage metadata from authoritative sources to be used for both the structured content authoring as well as the dossier management processes.
Solution Value Proposition
The InteliNotion platform maintains and tracks global dossier submissions, HA communications, artifacts, commitments, milestones and on-going submissions. The global visibility to regulatory information and submissions allows organizations to standardize global dossier management regulatory processes and achieve transformational improvements. The role-based access and visibility to full end-to-end dossier information provides significantly improved efficiencies.
The component-level management and traceability of all information across global dossier structures provides a content network, which is used to provide immediate and accurate change impact assessments and eliminates the need for time-consuming manual tracking. Regulatory planning can be improved by leveraging the platform capability to provide actionable dashboards of change impact assessments.