Solution Brief
Features
Solutions And Delivery Approach For Clinical
As part of our structured content authoring (SCA) solution, we deliver a clinical module by leveraging the core platform capabilities. Preconfigured base models and templates are delivered for the Protocol, Statistical Analysis Plan (SAP), and Clinical Study Report (CSR). Other templates that could be configured and delivered include a client-specific Study Outline or Summary, disclosure documents for study registration or results postings, Investigator’s Brochure, Common Technical Document (CTD) Summaries (e.g., Module 2.5, Module 2.7.3, Module 2.7.4), as well as Informed Consent Forms or other study -related documents.
Please refer to the Structured Content Authoring Solution Brief for general information concerning the patented InteliNotion platform and details concerning its specific available features.
Clinical Business Challenges
Clinical challenges described by our clients include:
- Manual copy/paste within and between documents results in no traceability
- Manual reuse of content across documents is time consuming
- Difficulty identifying related sets of content to reuse together (e.g., related sets of data tables that must be used together given a specific analysis method)
- Unable to trace what content was used in what document leading to potential quality and consistency issues across related study documents
- Unable to determine impact of change on up or downstream documents (e.g., if a protocol is amended, what other documents may be affected)
To help address these issues, as well as ensuring quality, consistency, and improved efficiencies, information can be structured within documents into meaningful content components that can be reused within and across documents while being fully traced. This structuring of information also aids in making and tracking key decisions during the authoring, review, and approval phases. Structuring content also promotes easier to track component level lifecycles and publishing of appropriate content components into required formats for downstream processes, systems, or applications.
Metadata Model Configurations
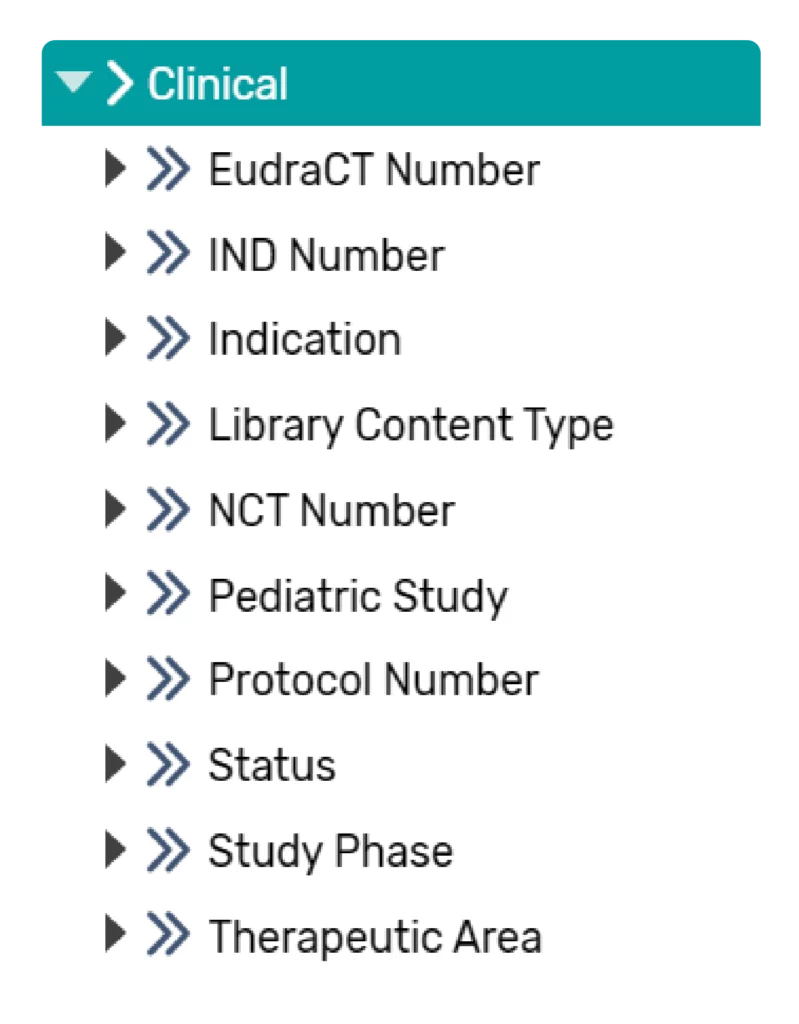
A baseline metadata catalogue will be supplied including standard product- study-level metadata elements that can be used for documents. The type and number of metadata are easily modified by the client to fit their needs. Configured metadata can be imported from authoritative source systems. Based on client-specific authoritative sources, integrations can be rapidly implemented within the platform. Examples of metadata that could be configured and used for clinical include:
Information Model Configurations
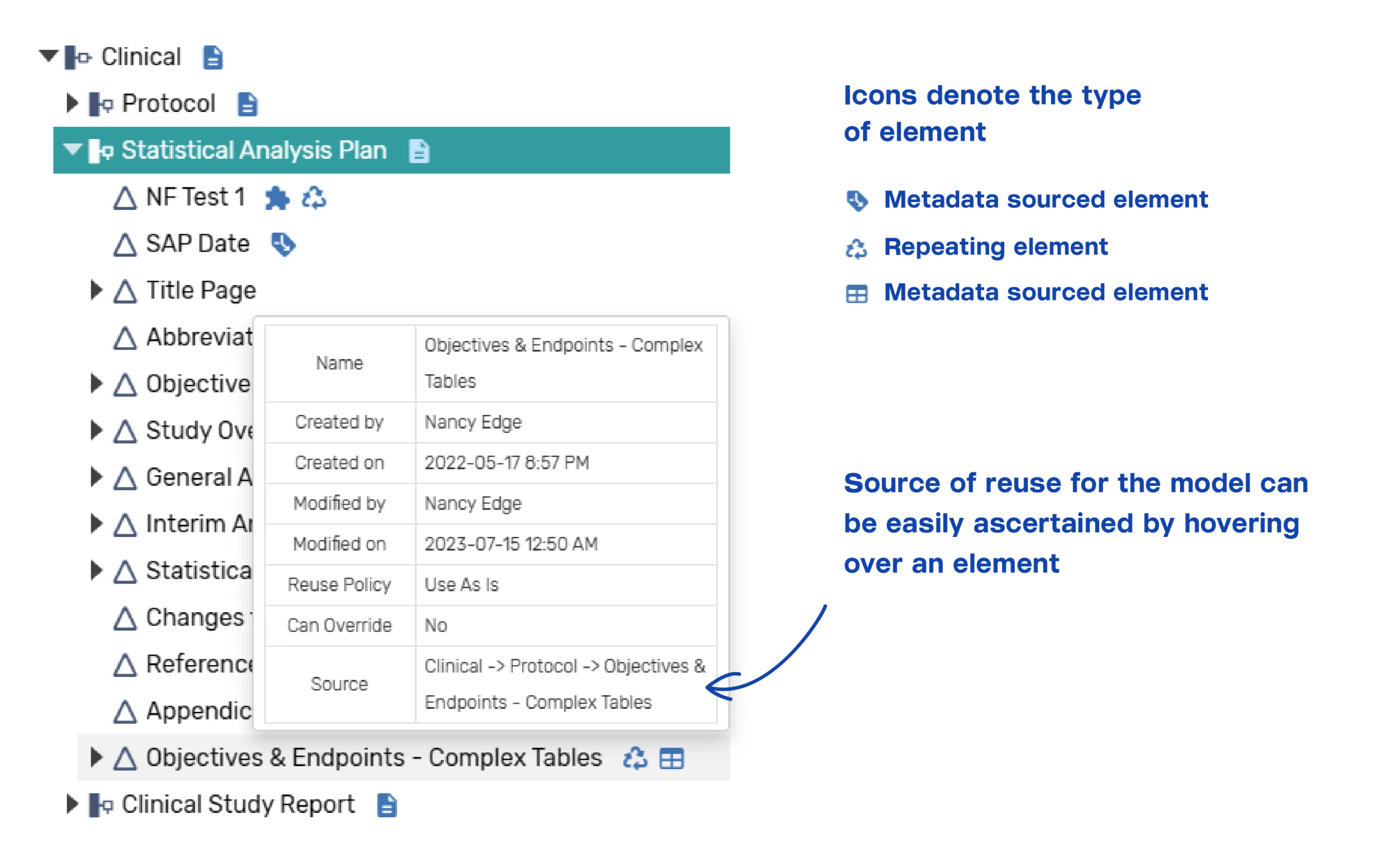
Baseline information models for clinical are provided for the protocol, SAP, and CSR. These models can be easily reconfigured by the client to accommodate any company-specific modifications or additions. Other updates that could be made include adjustment of the level of granularity for the component elements within a document, or the instructional text provided for each content component element. Information models for other clinical documents can be easily configured with our support or by the client using the baseline models as examples. A configured SAP and CSR models that reuses content across the documents is provided. The icons indicate whether the model’s element is for metadata or content, as well as if it is reused from another model.
Template Configurations
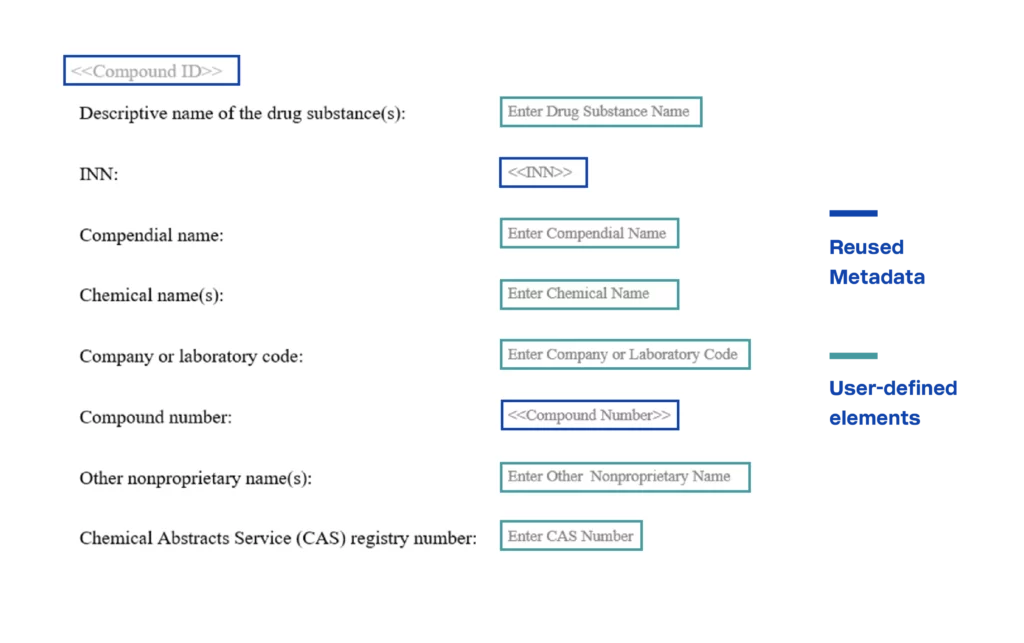
Baseline clinical templates have been configured following guidance documents and industry standards such as the TransCelerate Common Protocol Template (CPT) and ICH E3 Guidance. Instructional text can be added for each content component element to provide contextual information for authoring and reviewing that section, as well as reference to guidance documents, if desired. The templates are created by inserting content component and metadata elements defined by the business into a predefined structure (i.e., ordered sections). Multiple versions of any template can be stored if needed (e.g., a protocol template for interventional vs observational studies).
Document Generation & Authoring
Authorized users can easily generate clinical documents within the platform by simply selecting “Generate and Open Document” action and providing the clinical parameters (i.e., clinical document type) for the document to be generated. When the document is generated, the system will automatically:
- Reuse content from 1 or more existing documents based on reuse policies configured in the information models; reuse policies determine if content is reused “as-is” (no editing allowed) or repurposed (editing allowed in the new “branch” of the source component)
- For example, both a protocol and SAP can be used to generate a CSR
- Auto-populate content from content libraries based on configured rules; rules are prespecified within the information model and are executed based on user-selected metadata at the time of document generation
- For example, the protocol could be auto-populated with the appropriate adverse event intensity/severity language based on the corresponding therapeutic area
- Prepopulate variables (i.e. metadata values) based on template configurations
Authors can then edit the document by inserting ad hoc library content, by navigating to other documents to reuse known content, or by entering de novo content.
Regardless of how content is populated (reuse, auto-population, de novo), traceability of all components is maintained allowing for governance of the content and change impact assessment up or downstream via several reporting options.
Component/Document Review and Approval Process
In addition, document reviews and approvals are expedited because content can be reviewed or approved at either the component or document level. For example, a related set of content components (e.g., study assessments in the protocol) could be completed and then sent for review and approval early on in document development. Once the entire document is ready for review and approval, any pre-approved components are identified as such, so that reviewers and approvers can target the draft or non-approved content only.
Value Delivered
The InteliNotion platform facilitates an easy to configure approach that enables structured authoring for clinical documents. Information consistency between documents can be achieved by leveraging the use of library content, auto-population of prespecified content, and/or the reuse of metadata. Documents can also be generated with content from user-defined “base documents” that can be reused “as is” or with the ability to update the content, based on the pre-defined reuse policies. Throughout document authoring, traceability of the information is maintained. This traceability simplifies change impact assessment and identification of documents that may need to be updated. The notification system can be used to inform the organization of changes to documents, the potential need for updates, or any lifecycle change. All these functions can be defined with a lifecycle that is managed by the business for their unique needs. For day-to-day activities, features for authors and reviewers are provided in an App that allows users to work in the familiar Microsoft Word program with all its functions. Updating and maintaining the models and templates is intuitive and can be done by the business. Our team will provide training and business enablement.